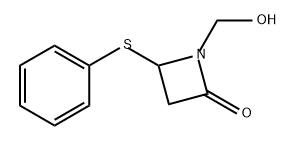
4-(PHENYLSULFANYL)AZETIDIN-2-ONE synthesis
- Product Name:4-(PHENYLSULFANYL)AZETIDIN-2-ONE
- CAS Number:31898-69-8
- Molecular formula:C9H9NOS
- Molecular Weight:179.24
Yield:31898-69-8 62%
Reaction Conditions:
with sodium hydrogencarbonate in water;acetone at 20; for 12 h;
Steps:
4.3.17. General procedure for synthesis of β-lactams containing aromatic thiols, phenol and thiophene
General procedure: Lactams 22 and 24, as well as 26-28 were prepared in a manner similar to the synthesis of lactam 9. 4-Acetoxy-2-azetidone (1 g, 8 mmol) was stirred in 50 mL acetone/water (3:2) and 1.05 equiv of the corresponding substituents (4-mercaptophenol, 4-aminothiophenol, 1,4-benzenedithiol, thiophenol, or 2-thiophene thiol, respectively) was added. Sodium bicarbonate (4 equiv) was added to the mixture, which was then stirred vigorously for 12 h in a closed round bottom flask. Sodium chloride was subsequently added to the solution and after the formation of two layers, the mixture was filtered out and extracted with AcOEt (3 × 50 mL). The combined organic layers were dried over MgSO4 and concentrated under vacuum. The crude material was purified by flash chromatography or crystallized to give white crystals for all five products in good yield (30-66%).
References:
Kostova, Maya B.;Myers, Carey J.;Beck, Tim N.;Plotkin, Balbina J.;Green, Jacalyn M.;Boshoff, Helena I.M.;Barry III, Clifton E.;Deschamps, Jeffrey R.;Konaklieva, Monika I. [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 22,p. 6842 - 6852] Location in patent:experimental part
97721-47-6
4 suppliers
inquiry

31898-69-8
6 suppliers
inquiry

28562-53-0
153 suppliers
$9.00/250mg
930-69-8
2 suppliers
$45.00/500mg

31898-69-8
6 suppliers
inquiry

934-87-2
110 suppliers
$41.15/5g

31898-69-8
6 suppliers
inquiry
![2-Azetidinone, 1-[(benzoyloxy)methyl]-4-(phenylthio)-](/CAS/20210305/GIF/344930-64-9.gif)