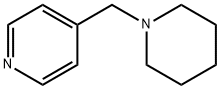
4-(Piperidin-1-ylmethyl)pyridine synthesis
- Product Name:4-(Piperidin-1-ylmethyl)pyridine
- CAS Number:34490-39-6
- Molecular formula:C11H16N2
- Molecular Weight:176.26
Yield:34490-39-6 98%
Reaction Conditions:
in methanol at 50; for 1 h;
Steps:
2.47.a
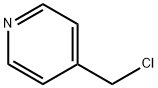
2.47.a 4-piperidin-1-ylmethyl-pyridine 242 mL piperidine (2.44 mol) are added dropwise to a solution of 100 g (0.61 mol) of 4-chloromethyl-pyridine in 600 mL dry methanol and the reaction mixture is stirred for one hour at 50° C. The solvent is eliminated using the rotary evaporator. The residue is made alkaline with 40% sodium hydroxide solution and the aqueous phase extracted with ether. The organic phase is dried over sodium sulphate and after filtration through activated charcoal the solvent is eliminated using the rotary evaporator. The crude product is further reacted without purification. Yield: 106 g (98% of theory).
References:
US2004/242572,2004,A1 Location in patent:Page/Page column 74