
(4-(Piperidin-1-ylsulfonyl)phenyl)MethanaMine synthesis
- Product Name:(4-(Piperidin-1-ylsulfonyl)phenyl)MethanaMine
- CAS Number:205259-71-8
- Molecular formula:C12H18N2O2S
- Molecular Weight:254.35
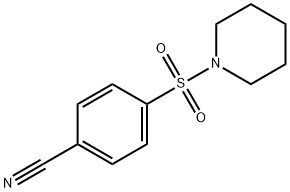
227935-30-0
9 suppliers
inquiry

205259-71-8
10 suppliers
$211.00/100mg
Yield:205259-71-8 25%
Reaction Conditions:
with ammonium hydroxide;hydrogen in methanol;water at 20; under 760.051 Torr;
Steps:
5.2 Step 2. 4-(Piperidine-1-sulfonyl)-benzylamine
To a solution of 4-(piperidine-1-sulfonyl)-benzonitrile (2 g, 7,83 mmol, 1.00 equiv) in methanol (40 mL) was added Raney Ni (0.2 g, 0. 10 equiv) and ammonium hydroxide (4 mL, 28-30% aqueous solution) The reaction mixture was stirred under 1 atmosphere of hydrogen overnight at rt, The catalyst was removed by filtration. The filtrate was concentrated under vacuum and the residue was purified on a silica gel column eluted with dichloromethane/methanol ( 1/30) to give 500 mg (25%) of the title compound as a light yellow oil. LC/MS (Method H, ESI): RT = 1 .08 mm, m/z = 255.0 [M + H]+ .
References:
WO2013/127266,2013,A1 Location in patent:Page/Page column 146; 147
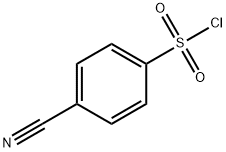
49584-26-1
158 suppliers
$21.21/1gm:

205259-71-8
10 suppliers
$211.00/100mg

110-89-4
3 suppliers
$15.96/100ML

205259-71-8
10 suppliers
$211.00/100mg