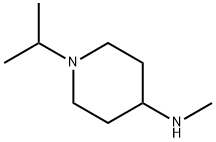
4-Piperidinamine,N-methyl-1-(1-methylethyl)-(9CI) synthesis
- Product Name:4-Piperidinamine,N-methyl-1-(1-methylethyl)-(9CI)
- CAS Number:503126-34-9
- Molecular formula:C9H20N2
- Molecular Weight:156.27
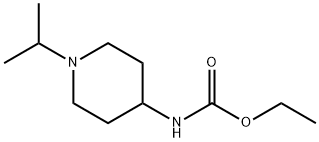
915373-91-0
1 suppliers
inquiry

503126-34-9
12 suppliers
$65.00/25mg
Yield:-
Reaction Conditions:
Stage #1: (1-isopropyl-piperidin-4-yl)-carbamic acid ethyl esterwith lithium aluminium tetrahydride in diethyl ether at 20; for 9 h;Heating / reflux;
Stage #2: with water in diethyl ether; for 0.333333 h;
Steps:
3.ii
ii) (1-Isopropyl-piperidin-4-yl)-methyl-amine 100 ml absolute diethyl ether were placed in a dry three-necked flask equipped with a condenser. 4.35 g LiAlH4 (6 equiv.) were added and the mixture was stirred at room temperature under an argon atmosphere. Carefully, 4.10 g (19.13 mmol) (1-isopropyl-piperidin-4-yl)-carbamic acid ethyl ester were added portionwise and the resulting mixture was refluxed for 7 h. Since the conversion was not complete,1.00 g (≈ 1.4 equiv.) LiAlH4 was added and the mixture was refluxed for further 2h. The reaction mixture was cooled and, carefully, 14 ml water were added dropwise over a 20-minute period. The mixture was diluted with 40 ml water and 50 ml diethyl ether. The phases were separated. The aqueous phase (suspension) was treated with a 10% NaOH-solution and washed twice with 100 ml diethyl ether. The organic phases were combined, dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude product was obtained as a colorless oil. Yield: 2.91 g MS (ES+): m/e = 157.
References:
EP1724269,2006,A1 Location in patent:Page/Page column 24
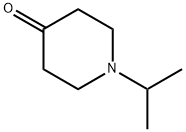
5355-68-0
202 suppliers
$9.00/1g

503126-34-9
12 suppliers
$65.00/25mg