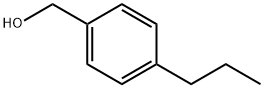
(4-propylphenyl)Methanol synthesis
- Product Name:(4-propylphenyl)Methanol
- CAS Number:82657-70-3
- Molecular formula:C10H14O
- Molecular Weight:150.22
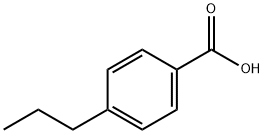
2438-05-3
160 suppliers
$11.00/10g

82657-70-3
20 suppliers
$220.00/100mg
Yield:82657-70-3 95%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran;diethyl ether at 0 - 20; for 1 h;Inert atmosphere;
Steps:
96.1 Step 1. Preparation of (4-propylphenyl)-methanol
Under nitrogen atmosphere, at 0 °C, to a suspension of LiA1H4 (2.0 M THF solution,6.1 mL, 12.2 mmol) in dry Et20 (30 mL), 4-propylbenzoic acid (0.5 g, 3.05 mmol) in dry Et20(10 mL) was added dropwise. The resulting reaction mixture was stirred at r.t. for 1 h, and thencooled to 0 °C. H20 (0.50 mL) was slowly and cautiously added, followed by 3.0 M KOHsolution (0.50 mL) and additional H20 (2.2 mL). The mixture was stirred at 0 °C for 1 h and then filtered off. The organic layer was dried over Na2504, filtered and concentrated to dryness, affording the title compound (0.43 g, 95%), which was used in the next step without any further purification. R= 2.22 mi ‘HNMR(DMSO-d6): ? 7.22 (d, 2H, J= 7.9 Hz), 7.13 (d, 2H, J= 7.9Hz), 5.07 (t, 1H, J= 5.8 Hz), 4.46 (d, 2H, J= 5.8 Hz), 2.53 (t, 2H, J= 7.5 Hz), 1.56 (sex, 2H, J=7.5 Hz), 0.89 (t, 3H, J= 7.5 Hz).
References:
WO2014/144836,2014,A2 Location in patent:Paragraph 0965; 0966
![Benzene, 1-[[[(1,1-dimethylethyl)dimethylsilyl]oxy]methyl]-4-propyl-](/CAS/20210305/GIF/1360803-32-2.gif)
1360803-32-2
0 suppliers
inquiry

82657-70-3
20 suppliers
$220.00/100mg
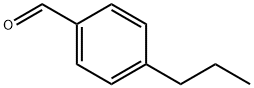
28785-06-0
111 suppliers
$45.00/500mg

82657-70-3
20 suppliers
$220.00/100mg
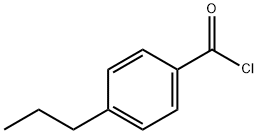
52710-27-7
80 suppliers
$17.67/1gm:

82657-70-3
20 suppliers
$220.00/100mg

1004992-92-0
0 suppliers
inquiry

82657-70-3
20 suppliers
$220.00/100mg