
4-Pyridinamine,3-bromo-N-methyl-(9CI) synthesis
- Product Name:4-Pyridinamine,3-bromo-N-methyl-(9CI)
- CAS Number:84539-38-8
- Molecular formula:C6H7BrN2
- Molecular Weight:187.04
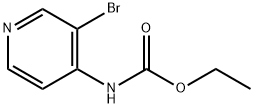
112671-56-4
1 suppliers
inquiry

84539-38-8
22 suppliers
$125.00/100mg
Yield:84539-38-8 30%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0;Reflux;
Steps:
3-Bromo-N-methylpyridin-4-amine (S8).
Ethyl (3-bromopyridin-4-yl)carbamate S7 (100 mol%) in THF (0.5 M) was added to a slurry ofLiAlH4 (150 mol%) in THF (0.5 M) at -78 °C. The mixture was stirred at 0 °C for 30 min,warmed to ambient temperature, and heated to reflux for additional 2-3 h. After completion, the mixture was cooled to 0 °C, diluted with CH2Cl2, and saturated acquous solution of Rochelle’ssalt. The pH was adjusted with acetic acid to 7. The organic layer was washed with water, brine.The combined aqueous layer was washed with CH2Cl2. The combined organic layer was driedover anhydrous Na2SO4, concentrated in vacuo. The product S8 was obtained as a yellow oil in 30% yield (233.1 mg, 1.54 mmol) after flash column chromatography (CHCl3/MeOH = 9:1). 1H NMR (400 MHz, CDCl3) δ 8.35 (d, J = 5.0 Hz, 1H), 8.17 (d, J = 5.5 Hz, 1H), 6.47 (t, J = 5.6Hz, 1H), 4.92 (s, 1H), 2.93 (m, 3H). 13C NMR (100 MHz, CDCl3) δ 151.1, 149.7, 148.4, 107.3, 105.4, 29.6. IR (neat) 3247, 2915, 2361, 1588, 1517, 1191, 1010 cm-1. HRMS (ESI+) calcd. for C6H8BrN2 [M+H]+ 186.9865, found 186.9860
References:
Shin, Inji;Ramgren, Stephen D.;Krische, Michael J. [Tetrahedron,2015,vol. 71,# 35,art. no. 26803,p. 5776 - 5780] Location in patent:supporting information
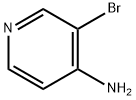
13534-98-0
276 suppliers
$6.00/250mg

84539-38-8
22 suppliers
$125.00/100mg