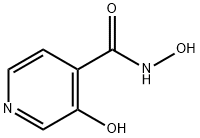
4-Pyridinecarboxamide, N,3-dihydroxy- synthesis
- Product Name:4-Pyridinecarboxamide, N,3-dihydroxy-
- CAS Number:89640-77-7
- Molecular formula:C6H6N2O3
- Molecular Weight:154.12
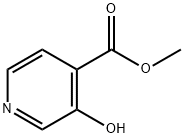
10128-72-0
105 suppliers
$11.00/250mg

89640-77-7
20 suppliers
$435.00/500mg
Yield: 96%
Reaction Conditions:
Stage #1:methyl 3-hydroxypyridine-4-carboxylate with sodium hydroxide;hydroxylamine hydrochloride in water at -5 - 20; for 1.5 h;
Stage #2: with hydrogenchloride in water; pH=4 - 5.4 at 5 - 60; for 1.5 h;
Steps:
A
Example A; 3, N-Dihydroxy-isonicotinamide To a stirred suspension of methyl 3-hydroxy-isonicotinate (176 g; 1.15 mol) in water/ice (50/50,1700 mL), was added hydroxylamine hydrochloride (127.9 g; 1.84 mol). The temperature fell to-5 °C and then aqueous NAOH solution (454 mL, 28% w/v) was added dropwise keeping the temperature below 5 °C during the addition. Hereafter the reaction mixture was stirred at ambient temperature for 1.5 h followed by heating to 60 °C. At this temperature the pH was adjusted to 5.4 by the addition of aqueous hydrochloric acid (10 M) at which point a heavy precipitate forms. The reaction mixture was then stirred at ambient temperature followed by cooling to 5 C. THE pH was then adjusted to 4.0 by the addition of aqueous hydrochloric acid (10 M), and then was stirred whilst cold for 1,5 h. The crystals were filtered off, rinsed with water (3 x 100 mL), dried on the filter and then dried further at reduced pressure and 40 °C overnight to give 3, N-dihydroxy-isonicotinamide (169.3 g, 96%; HPLC purity 98%) as a white solid. NMR data : H-NMR (DMSO-d6,250 MHz) 8 = 7.55 (1H, d, J=6 Hz); 8.11 (1H, d, J=6 Hz); 8.32 (1H, s); 9.56 (1H, s, broad peak); 11.50 (1H, s, broad peak) ppm.
References:
H. LUNDBECK A/S WO2005/23820, 2005, A1 Location in patent:Page/Page column 16
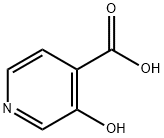
10128-71-9
192 suppliers
$8.00/1g

89640-77-7
20 suppliers
$435.00/500mg