
4-((Pyrimidin-2-ylamino)methyl)benzaldehyde synthesis
- Product Name:4-((Pyrimidin-2-ylamino)methyl)benzaldehyde
- CAS Number:405175-13-5
- Molecular formula:C12H11N3O
- Molecular Weight:213.24

897657-96-4
3 suppliers
inquiry

405175-13-5
4 suppliers
inquiry
Yield:-
Reaction Conditions:
with manganese(IV) oxide in dichloromethane;N,N-dimethyl-formamide at 20; for 19 h;
Steps:
16
To a solution of (4-((pyrimidin-2-ylamino)methyl)phenyl)methanol (prepared using the method of WO-2006-074428, herein incorporated by reference in its entirety) (4.4 g,
References:
WO2008/112156,2008,A1 Location in patent:Page/Page column 571-572
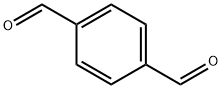
623-27-8
516 suppliers
$5.00/10g

405175-13-5
4 suppliers
inquiry