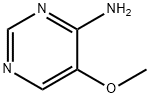
4-Pyrimidinamine, 5-methoxy- (9CI) synthesis
- Product Name:4-Pyrimidinamine, 5-methoxy- (9CI)
- CAS Number:695-86-3
- Molecular formula:C5H7N3O
- Molecular Weight:125.13
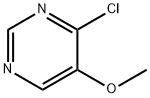
695-85-2
107 suppliers
$29.00/100mg

695-86-3
76 suppliers
$33.00/250mg
Yield: 87%
Reaction Conditions:
with ammonia in ethanol at 130; for 12 h;Autoclave;
Steps:
Step 4 - Synthesis of 4-amino-5-methoxypyrimidine (I-90)
A suspension of 4-chloro-5-methoxypyrimidine (I-89) (40 g, 280 mmol) in NH3 (g)/EtOH (4 M, 2000 mL) was poured into an autoclave at room temperature and stirred at 130 °C for 12 hr. TLC (CH2CI2/MeOH = 10:1 ) showed the reaction was complete. The mixture was cooled to room temperature and concentrated in vacuo to give a residue, to which was added CH2CI2 (100 mL) and the resulting mixture was stirred at room temperature for 30 min. The resulting suspension was filtered to remove NH4CI salt, and the filtrate was concentrated in vacuo to give the crude product. The crude product was stirred in a mixed solvent of EtOAc/CH2CI2 (50 mL, 4:1 ) for 30 min, filtered and concentrated in vacuo to obtain 4-amino-5-methoxypyrimidine (I-90) (30 g, 87%) as a yellow solid. 1H NMR (400 MHz, DMSO-c/6) 8 ppm 8.01 (s, 1 H), 7.81 (s, 1 H), 6.75 (br. s., 2 H), 3.81 (s, 3 H).
References:
PFIZER INC.;JOHNSON, Ted William;RICHARDSON, Paul Francis;COLLINS, Michael Raymond;RICHTER, Daniel Tyler;BURKE, Benjamin Joseph;GAJIWALA, Ketan;NINKOVIC, Sacha;LINTON, Maria Angelica;LE, Phuong Thi Quy;HOFFMAN, Jacqui Elizabeth WO2016/97918, 2016, A1 Location in patent:Page/Page column 80
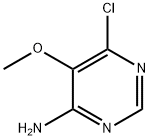
5018-41-7
146 suppliers
$39.00/100mg

695-86-3
76 suppliers
$33.00/250mg
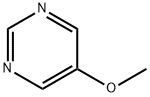
31458-33-0
93 suppliers
$26.00/100mg

695-86-3
76 suppliers
$33.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

695-86-3
76 suppliers
$33.00/250mg
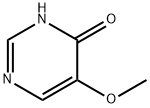
695-87-4
46 suppliers
$60.00/100mg

695-86-3
76 suppliers
$33.00/250mg