
4-Pyrimidinamine, 6-(2-ethoxyphenyl)- synthesis
- Product Name:4-Pyrimidinamine, 6-(2-ethoxyphenyl)-
- CAS Number:1142005-24-0
- Molecular formula:C12H13N3O
- Molecular Weight:215.25
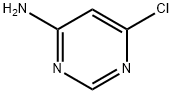
5305-59-9
321 suppliers
$8.00/5g
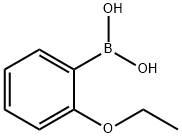
213211-69-9
205 suppliers
$8.00/1g

1142005-24-0
0 suppliers
inquiry
Yield:1142005-24-0 87.3%
Reaction Conditions:
with sodium carbonate;tetrakis(triphenylphosphine) palladium(0) in 1,4-dioxane;water; for 12 h;Heating / reflux;Suzuki Coupling;
Steps:
1A
To a solution of 2-ethoxyphenylboronic acid (II) (1.73 g, 10.4 mmol) in 30 ml of 1 , 4- dioxane 10 ml of saturated aqueous sodium carbonate solution were added. Argon gas was purged for 10 min at room temperature. 6-Chloro-pyhmidin-4-ylamine (I) (1.50 g, 11.5 mmol) and tetrakis(triphenylphosphine)palladium(0) (0.66 g, 0.57 mmol) were added to the reaction mixture simultaneously and argon gas was bubbled in for another 5 min. The reaction mixture was heated to reflux for 12 hours. After completion of the reaction as indicated by TLC the mixture was concentrated under reduced pressure. The residue was partitioned between dichloromethane and water. The organic layer was separated, washed with water and brine, dried over sodium sulfate and concentrated. The obtained crude residue was purified by column chromatography eluting with 15% ethyl acetate in dichloromethane to provide 6-(2-ethoxy-phenyl)- pyrimidin-4-ylamine (III) (2.17 g, 87.3%). 1H NMR (CDCI3) δ = 8.64 (1 H, s), 7.90-7.86 (1 H, m), 7.41 -6.88 (4H, m), 4.85 (2H, bs), 4.15 (2H, q), 1.40 (3H, t). MS: m/z = 216 (M+1 ).
References:
WO2009/47359,2009,A1 Location in patent:Page/Page column 138