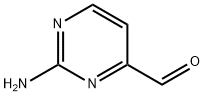
4-Pyrimidinecarboxaldehyde, 2-amino- (9CI) synthesis
- Product Name:4-Pyrimidinecarboxaldehyde, 2-amino- (9CI)
- CAS Number:165807-06-7
- Molecular formula:C5H5N3O
- Molecular Weight:123.11
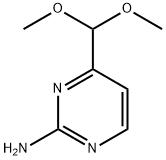
165807-05-6
104 suppliers
$19.00/100mg

165807-06-7
29 suppliers
inquiry
Yield:165807-06-7 37%
Reaction Conditions:
Stage #1:4-(dimethoxymethyl)pyrimidin-2-amine with hydrogenchloride;water at 48; for 14 h;
Stage #2: with water;sodium hydrogencarbonate
Steps:
A solution of 2.5 g (15 mmol, 1.0 eq.) 2-aminopyrimidine-4-carboxaldehyde dimethylacetal in 16 ml (45 mmol, 3 eq.) of 3M HCl was heated at 48° C. for 14 h. The mixture was cooled to r.t. and layered with 50 mL of EtOAc. The aqueous layer was neutralized with NaHCO3 and then extracted with EtOAc (5*50 ml). The combined extracts were dried (Na2SO4) and concentrated in vacuo afford 0.69 g (37%) of 2-aminopyrimidine-4-carboxaldehyde. 1H NMR (300 MHz, CDCl3): δ 9.82 (s, 1H), 8.75 (d, 1H), 7.10 (d, 1H), 5.28 (s, 2H) ppm.
References:
PHARMACOPEIA, INC. US2008/85898, 2008, A1 Location in patent:Page/Page column 17
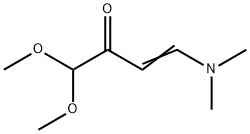
67751-23-9
92 suppliers
$25.00/1g

165807-06-7
29 suppliers
inquiry