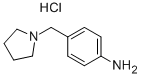
4-(PYRROLIDIN-1-YLMETHYL)ANILINE HYDROCHLORIDE synthesis
- Product Name:4-(PYRROLIDIN-1-YLMETHYL)ANILINE HYDROCHLORIDE
- CAS Number:866956-98-1
- Molecular formula:C11H17ClN2
- Molecular Weight:212.72
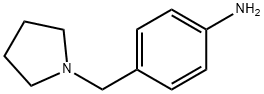
142335-64-6
54 suppliers
$60.00/100mg

866956-98-1
34 suppliers
$240.00/1g
Yield:866956-98-1 61%
Reaction Conditions:
with hydrogenchloride in methanol;diethyl ether;pentane;
Steps:
8.1
Step 1. Preparation of 4-(pyrrolidin-l-ylmethyl)aniline (HC1 salt):To l-(bromomethyl)-4-nitrobenzene (5 g, 23.1 mmol) dissolved in 100 mL of THF was added pyrrolidine (2.28 mL, 27.72 mmol) followed by K2C03 (4.79g, 34.65 mmol) was stirred at room temperature overnight. The resulting mixture was filtered to remove the salt, cocnentrated fitrate to dryness and pumpled under high vacuum fro 25 minutes to obtain l-(4-nitrobenzyl)pyrrolidine as a red oil (4.75 g) Pd/C (approximately 300 mg) was added and was hydrogenated over 1 atm H2 overnight in EtOH (100 mL). The resulting mixture was filtered through a pad of celite and conentrated to afford the crude 4-(pyrrolidin-l-ylmethyl)aniline as a yellow oil. All attempts to crystallize from pentane:ether would not produce a solid. Therefore, the yellow oil was suspended in diethyl ether, added 2 equiv. HC1 (1.25 M in MeOH) and added pentane. The resulting precipitated salt was collected and dried to obtain a yellow oil consisting of 4- (pyrrolidin- 1 -ylmethyl)aniline as the HC1 salt (2.98 g, 61% yield).
References:
WO2011/116176,2011,A1 Location in patent:Page/Page column 86-87
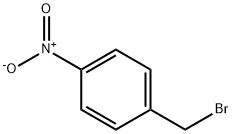
100-11-8
9 suppliers
$13.00/5g

866956-98-1
34 suppliers
$240.00/1g
![1-[(4-Nitrophenyl)Methyl]pyrrolidine](/CAS/GIF/133851-67-9.gif)
133851-67-9
21 suppliers
$60.00/50mg

866956-98-1
34 suppliers
$240.00/1g