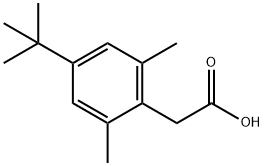
4-tert-Butyl-2,6-dimethyl-alpha-toluic acid synthesis
- Product Name:4-tert-Butyl-2,6-dimethyl-alpha-toluic acid
- CAS Number:854646-92-7
- Molecular formula:C14H20O2
- Molecular Weight:220.31
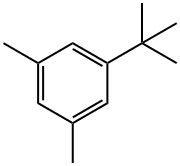
98-19-1
170 suppliers
$15.00/25g

64-19-7
1540 suppliers
$10.00/25ML

298-12-4
449 suppliers
$5.00/25g

854646-92-7
25 suppliers
inquiry
Yield:854646-92-7 86 %Chromat.
Reaction Conditions:
Stage #1: 5-tert-Butyl-m-xylene;acetic acid;Glyoxilic acidwith sulfuric acid in water at 20 - 60; for 9 h;
Stage #2: with hydrogenchloride;phosphorus;acetic acid;potassium iodide in water at 100; for 16 h;
Steps:
1; 4
A mixture of 89 g of 50% aqueous glyoxylic acid solution [0.6 mol], 400 ml of glacial acetic acid and 81.1 g [0.5 mol] of 1-tert-butyl-3,5-dimethylbenzene is initially charged. Starting at room temperature, 85.8 g of 96% strength sulfuric acid [0.84 mol] are added dropwise over a period of 15 minutes, during which time the temperature of the reaction mixture increases to about 35° C. The mixture is heated to 60° C. and stirred at this temperature for 9 hours. The cooled reaction mixture is then stirred into 750 ml of ice-water. The mixture is extracted three times with in each case 150 ml of methylene chloride, the combined organic phases are washed with 100 ml of saturated aqueous NaCl solution, dried over sodium sulfate and concentrated under reduced pressure. This gives 136.7 g of a yellowish thick oil, which, according to GC/MS(sil.), has the following composition: 2.6 area% of 1-tert-butyl-3,5-dimethylbenzene (4.4% of the starting material employed) 23.7 area% of 4-tert-butyl-2,6-dimethylmandelic acid (27.4% of theory) 67.2 area% of 4-tert-butyl-2,6-dimetlylmandelic acid acetate (66% of theory)A mixture of 2.89 g of 4-tert-butyl-2,6-dimethylmandelic acid and 7.75 g of 4-tert-butyl-2,6-dimethylmandelic acid acetate, 4.5 g of 37% strength hydrochloric acid, 1.86 g of red phosphorus and 0.66 g of KI in 30 ml of glacial acetic acid is heated at 100° C. for 16 hours. Excess phosphorus is filtered off with suction and washed three times with in each case 10 ml of glacial acetic acid. The filtrate is substantially concentrated on a rotary evaporator at a bath temperature of 50° C/60 mbar. The resulting residue is diluted with 25 ml of water and, by addition of 10% strength aqueous sodium hydroxide solution, dissolved. This solution is extracted twice with in each case 20 ml of MTBE and then adjusted to pH 1 using 48% strength sulfuric acid. The resulting greasy solid is taken up in methylene chloride. This solution is extracted with 25 ml of water, dried over sodium sulfate and concentrated using a rotary evaporator. This gives 7.66 g of 4-tert-butyl-2,6-dimethylphenylacetic acid in a purity of 99.0 GC area% (yield about 86% of theory).
References:
US2012/116118,2012,A1 Location in patent:Page/Page column 7-8

1260490-37-6
2 suppliers
inquiry

854646-92-7
25 suppliers
inquiry

98-19-1
170 suppliers
$15.00/25g

854646-92-7
25 suppliers
inquiry

108-38-3
352 suppliers
$10.60/50gm:

854646-92-7
25 suppliers
inquiry
84803-57-6
41 suppliers
inquiry

854646-92-7
25 suppliers
inquiry