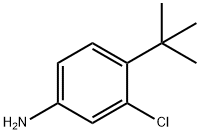
4-tert-butyl-3-chlorobenzenamine synthesis
- Product Name:4-tert-butyl-3-chlorobenzenamine
- CAS Number:52756-36-2
- Molecular formula:C10H14ClN
- Molecular Weight:183.68
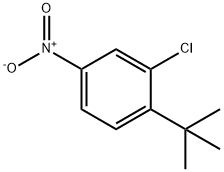
52756-38-4
12 suppliers
inquiry

52756-36-2
41 suppliers
inquiry
Yield: 99%
Reaction Conditions:
with sodium tetrahydroborate;nickel dibromide in tetrahydrofuran;methanol at 0 - 35; for 1 h;
Steps:
9.7 (Step 7)
(Step 7)
To a solution of 1-(tert-butyl)-2-chloro-4-nitrobenzene (8.57 g, 40.11 mmol) and nickel(II) bromide (0.438 g, 2.01 mmol) in a mixed solvent of MeOH (170 mL) and THF (170 mL) was added sodium borohydride (4.55 g, 120.33 mmol) at 0°C, and the mixture was stirred at 0°C for 20 min, and then at room temperature for 40 min.
To the reaction mixture was added aqueous sodium hydrogencarbonate solution, and the mixture was extracted with ethyl acetate (x3).
The organic layer was dried over magnesium sulfate, and the solvent was evaporated under reduced pressure.
The obtained residue was purified by silica gel column chromatography (solvent gradient; 4→20% ethyl acetate/hexane) to give 4-(tert-butyl)-3-chloroaniline (7.30 g, 39.7 mmol, 99%) as a colorless oil.
1H NMR(300
MHz,CDCl3) :δ1.43(9H,s), 3.58(2H,brs), 6.51(1H,dd,J=8.7,2.6 Hz), 6.71(1H,d,J=2.6 Hz), 7.18 (1H,d,J=8.7 Hz).
References:
Takeda Pharmaceutical Company Limited;YAMAMOTO, Satoshi;SHIRAI, Junya;KONO, Mitsunori;TOMATA, Yoshihide;SATO, Ayumu;OCHIDA, Atsuko;FUKASE, Yoshiyuki;FUKUMOTO, Shoji;ODA, Tsuneo;TOKUHARA, Hidekazu;ISHII, Naoki;SASAKI, Yusuke EP3018123, 2016, A1 Location in patent:Paragraph 0482

103392-84-3
28 suppliers
inquiry

52756-36-2
41 suppliers
inquiry
6310-21-0
217 suppliers
$6.00/1g

52756-36-2
41 suppliers
inquiry

3282-56-2
74 suppliers
$11.00/1g

52756-36-2
41 suppliers
inquiry