
4-(TERT-BUTYL)-5-CHLOROPYRIMIDIN-2-AMINE synthesis
- Product Name:4-(TERT-BUTYL)-5-CHLOROPYRIMIDIN-2-AMINE
- CAS Number:1095823-83-8
- Molecular formula:C8H12ClN3
- Molecular Weight:185.65
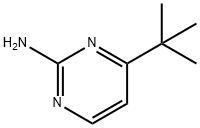
17321-94-7
15 suppliers
$45.00/10mg

1095823-83-8
6 suppliers
inquiry
Yield:1095823-83-8 81%
Reaction Conditions:
with N-chloro-succinimide in chloroform; for 1.5 h;Heating / reflux;
Steps:
H
Synthesis of Compound H.3. A solution of compound H.2 (200 mg, 1.32 mmol) and N-chlorosuccinimide (185 mg, 1.39 mmol) in chloroform (3.4 mL) was refluxed. After 1.5 hr, sat. aq. NaHCO3 and EtOAc were added. The aqueous layer was extracted three times with EtOAc. The combined organic layers were washed with brine, dried over anhydrous sodium sulfate, filtered, and concentrated. The crude product was purified by flash chromatography (hexanes/EtOAc=5:1→3:1) to afford 200 mg (81%) of compound H.3 as a white solid. 1H NMR (400 MHz, MeOD-d4): δ8.02 (s, 1H), 1.40 (s, 9H); MS: m/z 186 [M+1]+.
References:
US2009/5359,2009,A1 Location in patent:Page/Page column 38