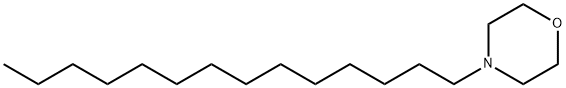
4-tetradecylmorpholine synthesis
- Product Name:4-tetradecylmorpholine
- CAS Number:25727-93-9
- Molecular formula:C18H37NO
- Molecular Weight:283.49
Yield:25727-93-9 93%
Reaction Conditions:
with sodium hydroxide in water at 130; for 7 h;
Steps:
3.1. Synthesis of BMTB
The preparation of morpholine-typed Gemini surfactant BMTB is shown in Fig. 3. 1-Bromotetradecane (1; 27.73 g, 0.1 mol) was mixed in a reactor with NaOH (4.8 g, 0.12 mol) at 130 °C for 30 min. Then, an aqueous solution of morpholine (2; 10.455 g, 0.12 mol) was injected slowly. The reaction time is 7 h. Then, cooling down the mixture to 20 °C leaded to two liquid phase separation. The top phase contained the product, dried to remove water and produced the a chromatous oil which was N-(n-Tetradecyl) morpholine (3; 26.32 g, 0.093 mol, 93%yield). The obtained N-(n-Tetradecyl) morpholine (3, 16.98 g, 0.06 mol) and 1,4-dibromobutane (4, 5.40 g, 0.025 mol) were mixed and set into a reactor containing ethanol (50 mL), and refluxed for 3 days. The obtained product butanediyl-α,ω-bis(morpholinotetradecylammonium bromide) (5, 14.645 g, 0.0185 mol) was purified through solvent evaporation, washing four times by 200 mL of ethyl acetate, recrystallization in mixed solvent (9/1 v/v ethyl acetate/ethanol) and vacuum drying.
References:
Ai, Guanghua;Cheng, Chen;Fu, Weng;Guo, Zhiqun;He, Guichun;Huang, Zhiqiang;Liu, Rukuan;Liu, Zuwen;Wang, Hongling;Yu, Xinyang;Zhang, Feng;Zhang, Shiyong;Zhou, Jianrong [Journal of Molecular Liquids,2020,vol. 313]
5338-53-4
2 suppliers
inquiry

25727-93-9
7 suppliers
inquiry

