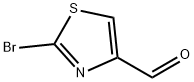
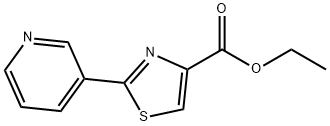
4-Thiazolecarboxaldehyde, 2-(3-pyridinyl)- synthesis
- Product Name:4-Thiazolecarboxaldehyde, 2-(3-pyridinyl)-
- CAS Number:184679-13-8
- Molecular formula:C9H6N2OS
- Molecular Weight:190.22
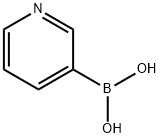
39067-28-2
21 suppliers
$317.00/250mg

184679-13-8
2 suppliers
inquiry
Yield:184679-13-8 71%
Reaction Conditions:
Stage #1: ethyl 2-(pyridin-3-yl) thiazole-4-carboxylatewith diisobutylaluminium hydride in dichloromethane at -78; for 3 h;
Stage #2: with methanol in dichloromethane at -78 - 25;
Stage #3: with water;Rochelle's salt in dichloromethane at 25; pH=7; for 16 h;
Steps:
19.B
A solution containing the product from Example 19A (20 g, 85.5 mmol) in dichloromethane (340 mL) was treated dropwise with DIBAL (86 mL, 1 M in dichloromethane) at -78° C., stirred at -78° C. for 2 hours, treated with DIBAL (43 mL, 1 M in dichloromethane), stirred at -78° C. for 1 hour, treated with methanol (20 mL) at -78° C., warmed to 25° C., treated with dichloromethane (250 mL), saturated aqueous sodium potassium tartrate (350 mL), and pH 7 buffer (300 mL), stirred vigorously with a mechanical stirrer for 16 hours, and filtered through celite. The aqueous phase was washed with chloroform, and the combined organic phase were washed with brine and dried over MgSO4, filtered and concentrated. The residue was chromatographed on silica gel eluting 0-4% methanol/chloroform to give the title compound (11.61 g, 71% yield).
References:
US2005/131017,2005,A1 Location in patent:Page/Page column 107