
4'-(trifluoromethoxy)propiophenone synthesis
- Product Name:4'-(trifluoromethoxy)propiophenone
- CAS Number:94108-55-1
- Molecular formula:C10H9F3O2
- Molecular Weight:218.17
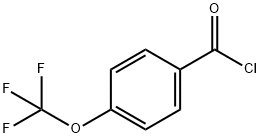
36823-88-8
145 suppliers
$10.00/1g

2386-64-3
208 suppliers
$45.00/5ml

94108-55-1
17 suppliers
$265.00/2.5g
Yield:94108-55-1 93%
Reaction Conditions:
with iron(III)-acetylacetonate in tetrahydrofuran at -20; for 1 h;Inert atmosphere;
Steps:
1
[0126] A solution of ethylmagnesium chloride (2 M in THF, 9.38 mL, 18.77 mmol) was added overmm to a solution of 4-(trifluoromethoxy)benzoyl chloride (11-1) (purity 98%, 5.00 g, 21.82 mmol)iron(III) acetylacetonate (385 mg, 1.09 mmol) in THF (70 mL) at -20°C under argon. After stirring10 mm at that temperature, the reaction mixture was quenched with 1 N HC1 and the mixture wasrepeatedly extracted with CH2C12. The combined organic layers were washed with 2 N NaOH, driedover Na2SO4 and evaporated to yield 1-[4-(trifluoromethoxy)phenyl]propan-1-one (111-1) (purity 90%,4.95 g, 93%). ‘H NMR (400 MHz, DMSO-d6) 1.09 (t, 3H), 3.07 (q, 2H), 7.50 (d, 2H), 8.10 (d, 2H).
References:
WO2015/71260,2015,A1 Location in patent:Paragraph 0126
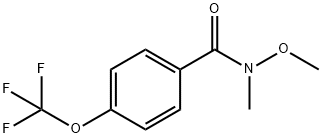
897656-36-9
11 suppliers
inquiry
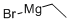
925-90-6
344 suppliers
$12.00/5ml

94108-55-1
17 suppliers
$265.00/2.5g
330-12-1
238 suppliers
$15.00/10g

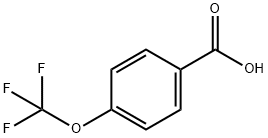
94108-55-1
17 suppliers
$265.00/2.5g

36823-88-8
145 suppliers
$10.00/1g

94108-55-1
17 suppliers
$265.00/2.5g