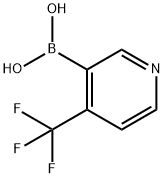
4-Trifluoromethyl-pyridine-3-boronic acid synthesis
- Product Name:4-Trifluoromethyl-pyridine-3-boronic acid
- CAS Number:947533-41-7
- Molecular formula:C6H5BF3NO2
- Molecular Weight:190.92

5419-55-6
369 suppliers
$12.00/5g
936841-70-2
99 suppliers
$19.00/1g

947533-41-7
32 suppliers
$45.00/10mg
Yield: 15%
Reaction Conditions:
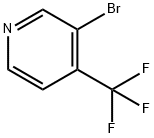
Stage #1:Triisopropyl borate;3-bromo-4-(trifluoromethyl)pyridine with n-butyllithium in tetrahydrofuran at -78 - 20;Inert atmosphere;
Stage #2: with water in tetrahydrofuran
Steps:
75
Example 75r4-(trifluoromethyl)-3-pyridyllboronic acidn-Butyllithium (2.5 M in hexane, 2 mL) is added to a mixture of 3-bromo-4- (trifluoromethyl)pyridine (Matrix Scientific, 1 g, 0.022 mol) and triisopropylborate (1.25 mL) in anhydrous THF (9 mL) at -78 °C under nitrogen. The reaction mixture is stirred at -78 °C for 3.5 hr before warming gradually to room temperature. The reaction is quenched with water (9 mL). The organic solvent is removed under reduced pressure. The resulting aqueous phase is treated with NaOH (10 N) to obtain pH 10, washed with diethyl ether (1 x 8 mL), and aqueous phase is acidified to pH 5 using acetic acid. The solution is extracted with EtOAc (1 x 25 mL) and the organic layer is dried and evaporated to dryness to give the title compound (0.150 g, 15% yield). LCMS m/z = 192.2 [M+H]+, fR = 0.36 min.
References:
PAMLICO PHARMACEUTICAL INC.;ATKINSON, Robert N.;OMMEN, Andy J.;VEAL, James M.;HUANG, Kenneth H.;SMITH, Emilie, D. WO2012/88411, 2012, A1 Location in patent:Page/Page column 69