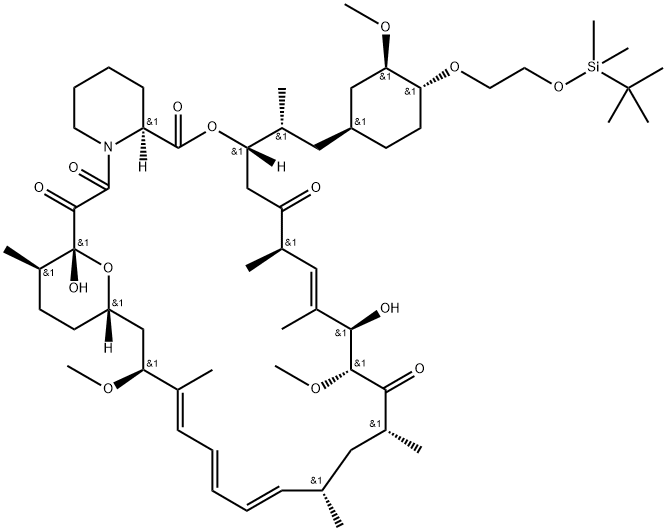
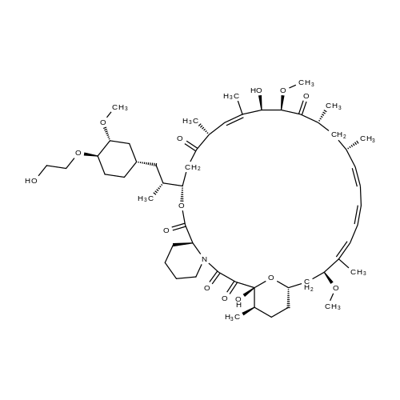
40-O-[2-(t-butyldimethylsilyl)oxy]ethyl rapamycin synthesis
- Product Name:40-O-[2-(t-butyldimethylsilyl)oxy]ethyl rapamycin
- CAS Number:159351-68-5
- Molecular formula:C59H97NO14Si
- Molecular Weight:1072.5
75-21-8
217 suppliers
$33.29/crm44609
69739-34-0
357 suppliers
$10.00/1g

53123-88-9
759 suppliers
$9.00/10mg
![40-O-[2-(t-butyldimethylsilyl)oxy]ethyl rapamycin](/CAS/20211123/GIF/159351-68-5.gif)
159351-68-5
16 suppliers
inquiry
Yield:159351-68-5 76 %Chromat.
Reaction Conditions:
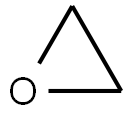
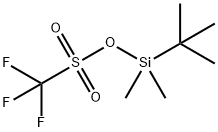
Stage #1:oxirane;t-butyldimethylsiyl triflate with N-ethyl-N,N-diisopropylamine in toluene at 20; for 0.666667 h;
Stage #2:sirolimus with N,N-diisopropylethylamine trifluoromethanesulfonate in 1,2-dimethoxyethane;toluene at 48 - 50; for 18 h;Inert atmosphere;
Steps:
1 Example 1 : Synthesis of 40-O-[2-(f-butyldimethylsilyl)oxy]ethyl-rapamycin
Example 1 : Synthesis of 40-O-[2-(f-butyldimethylsilyl)oxy]ethyl-rapamycin A mixture of 7.9 ml of 1 M ethylene oxide solution in toluene and 1.01 g of Λ/,/ν-diisopropyl- ethylamine was added under stirring to 1.72 g of f-butyldimethylsilyl trifluoromethane- sulfonate. The resulting mixture was stirred for further 40 min at 20°C. Then, 0.66 ml of 1 ,2- dimethoxyethane and 1.82 g of W,/v-diisopropylethylamine trifluoromethanesulfonate were added. The resulting mixture was stirred at 20°C until complete dissolution, before 1 .0 g of rapamycin was added. The reaction mixture was purged with nitrogen for 10 min, heated to 48-50°C and stirred at this temperature for 18 h. Subsequently, the reaction mixture was cooled to 20°C, and 0.2 ml of pyridine and 16 ml of heptane were added. The resulting mixture was stirred for 10 minutes. The precipitate formed was obtained by filtration and washed with a mixture of 4.0 ml of toluene and 9.4 ml of heptane. Then, 1 ml of 0.2% solution of 2,6-di-f-butyl-4-methylphenol in heptane was added to the filtrate and the resulting crude 40-O-[2-(f-butyldimethylsilyl)oxy]ethyl-rapamycin solution was subjected to the deprotection step. HPLC analysis of the obtained 40-O-[2-(f-butyldimethylsilyl)oxy]ethyl- rapamycin solution showed an overall yield of 76% (895 mg).
References:
SYNBIAS PHARMA AG;ZABUDKIN, Oleksandr;SCHICKANEDER, Christian;MATVIIENKO, Iaroslav;SYPCHENKO, Volodymyr WO2016/207205, 2016, A1 Location in patent:Page/Page column 12; 13

53123-88-9
759 suppliers
$9.00/10mg
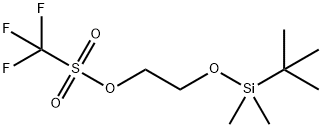
164162-36-1
58 suppliers
inquiry
![40-O-[2-(t-butyldimethylsilyl)oxy]ethyl rapamycin](/CAS/20211123/GIF/159351-68-5.gif)
159351-68-5
16 suppliers
inquiry
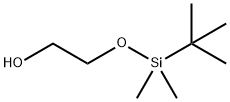
102229-10-7
172 suppliers
$10.00/1g

53123-88-9
759 suppliers
$9.00/10mg
![40-O-[2-(t-butyldimethylsilyl)oxy]ethyl rapamycin](/CAS/20211123/GIF/159351-68-5.gif)
159351-68-5
16 suppliers
inquiry
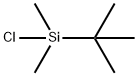
18162-48-6
668 suppliers
$9.00/5g
159351-69-6
502 suppliers
$8.00/1mg
![40-O-[2-(t-butyldimethylsilyl)oxy]ethyl rapamycin](/CAS/20211123/GIF/159351-68-5.gif)
159351-68-5
16 suppliers
inquiry

102229-10-7
172 suppliers
$10.00/1g
![40-O-[2-(t-butyldimethylsilyl)oxy]ethyl rapamycin](/CAS/20211123/GIF/159351-68-5.gif)
159351-68-5
16 suppliers
inquiry