
7 β - aMino - 3 - [4 - pyridyl - 2 - thiazole sulfur radical ] - 3 - cepheM - 4 - carboxylic acid ·2HCl synthesis
- Product Name:7 β - aMino - 3 - [4 - pyridyl - 2 - thiazole sulfur radical ] - 3 - cepheM - 4 - carboxylic acid ·2HCl
- CAS Number:400827-64-7
- Molecular formula:C16H16Cl2N4O3S3
- Molecular Weight:479.4242
Yield: 74.3%
Reaction Conditions:
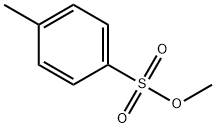
Stage #1:methyl p-toluene sulfonate;4-(2-{[(6R,7R)-2-[(diphenylmethoxy)carbonyl]-8-oxo-7-(2-phenylacetamido)-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]sulfanyl}-1,3-thiazol-4-yl)pyridine in dichloromethane for 12 h;Reflux;
Stage #2: with pyridine;phosphorus pentachloride in dichloromethane at -10 - -5; for 2 h;
Stage #3: with hydrogenchloride in dichloromethane;2-methyl-propan-1-ol;acetone at 25 - 30;
Steps:
4
A glass round bottom flask was charged with 35g (51,7 mmol) of 5-Thia-1-azabicyclo[4.2.0]oct- 2-ene-2-carboxylic acid, 8-oxo-7-[(2-phenylacetyl)amino]-3-[[4-(4-pyridinyl)-2-thiazolyl]thio]-, diphenylmethyl ester, (6R,7R) and 75 mE of dichloromethane, at 25°C. To the obtained solution were added 48 mE (258,5 mmol) of methyl paratoluenesulphonate and the mixture was heated to reflux and maintained for 12 hours under reflux, then cooled to room temperature. In glass lined reactor were charged 32,3 g (155,1 mmol) of phosphorous pentachloride and 75 mE of dichloromethane. The obtained suspension was cooled to -5÷0°C and 12.3 g (155,1 mmol)of pyridine were added dropwise by maintaining the temperature at -5÷0°C, then the mixture was maintained under stifling for 15 minutes at the same temperature. To the mixture, cooled to -1 0÷-5°C, was carefully added the solution prepared in the round bottom flask, keeping the temperature between -1 0÷-5°C; the mixture thus obtained was maintained under stifling for 2 hours at the same temperature. 105 mE of isobutanol were carefully added, in about 30 minutes, by maintaining the temperature at -5°C0°C; the obtained mixture was heated at 28÷30°C and left to react at the same temperature for 3.5 hours. The mixture was concentrated under vacuum to 1/3 of its volume, diluted with 200 mE of acetone and maintained under stifling for 1 hour at 25÷30°C. 70 mE of 32% hydrochloric acid were added, keeping the temperature in the range of 25÷30°C, and themixture was maintained under stirring overnight. The obtained suspension was filtered, the cake was washed with acetone (2 x 35 ml). The wet solid was dried under vacuum at 40°C overnight yielding 18.4 g (38,4 mmol) of 4-[2- [[(6R,7R)-7-amino-2-carboxy-8-oxo-5-thia- 1- azabicyclo [4.2.01 oct-2-en-3-yl]thio] -4-thiazolyl] -1 -methyl pyridinium, chloride, hydrochloride (1:1:1) as a crystalline solid. Yield 74,3%.
References:
FRESENIUS KABI ANTI-INFECTIVES SRL;RICCI, Antonio;ZANON, Jacopo WO2016/128580, 2016, A1 Location in patent:Page/Page column 21
![Diphenylmethyl (6R,7R)-8-oxo-7-[(phenylacetyl)amino]-3-{[4-(pyridin-4-yl)-1,3-thiazol-2-yl]thio}-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate](/CAS/20200119/GIF/400827-68-1.gif)