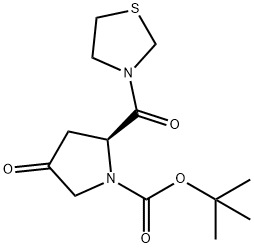
(2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester synthesis
- Product Name:(2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester
- CAS Number:401564-36-1
- Molecular formula:C13H20N2O4S
- Molecular Weight:300.37
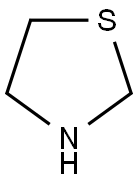
504-78-9
227 suppliers
$16.00/1g
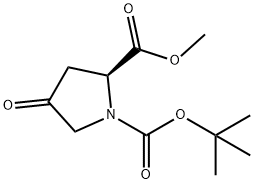
102195-80-2
238 suppliers
$5.00/250mg

401564-36-1
233 suppliers
$6.00/1g
Yield:401564-36-1 90%
Reaction Conditions:
with acetic acid in methanol at 0 - 30; for 2.5 h;Large scale;
Steps:
4 The specific synthesis process 4, the synthesis of compound 4-1
Add 1500 Kg of methanol to the reaction kettle.250Kg compound 3-1, acetic acid 6.4Kg,Then, the temperature was lowered to 0 to 5 ° C, and then 96 kg of tetrahydrothiazole was added dropwise to the reaction vessel at an internal temperature of not more than 10 °C.After the completion of the dropwise addition, the reaction was kept at 0 to 5 ° C for 2 h, and then raised to 30 ° C for 0.5 h.After the reaction is completed, the mixture is concentrated under reduced pressure until solvent-free evaporation.Add 1500 Kg of dichloromethane, 300 Kg of saturated sodium bicarbonate solution to the reaction kettle, and stir for 30 minutes.After standing for 30 minutes, the layers were separated, and the organic layer was washed twice with 150 Kg of brine.The organic phase was concentrated under reduced pressure to give a pale yellow solid.The crude product was then dissolved in 280 kg of ethyl acetate.Heat to 50 ~ 55 ° C, dissolve, add 1120Kg petroleum ether to the reaction kettle, add dropwise,Slowly cool to 0 ~ 5 ° C, and crystallization at 0 ~ 5 ° C for 30 minutes.After centrifugation, the solid was washed with a petroleum ether: ethyl acetate (4:1) mixed solution of 100 Kg.Drying at 45 to 50 ° C gives 278 Kg of compound 4-1.The yield was 90%.
References:
CN110294748,2019,A Location in patent:Paragraph 0034-0036

504-78-9
227 suppliers
$16.00/1g
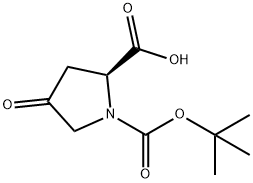
84348-37-8
275 suppliers
$5.00/250mg

401564-36-1
233 suppliers
$6.00/1g
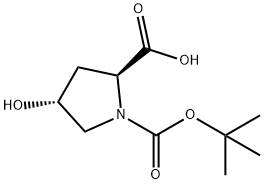
13726-69-7
429 suppliers
$5.00/1g

401564-36-1
233 suppliers
$6.00/1g

24424-99-5
824 suppliers
$13.50/25G

401564-36-1
233 suppliers
$6.00/1g
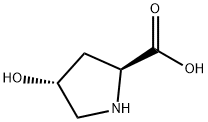
51-35-4
838 suppliers
$6.00/5g

401564-36-1
233 suppliers
$6.00/1g