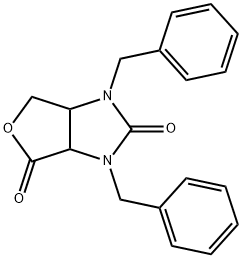
1H-Furo[3,4-d]imidazole-2,4-dione, tetrahydro-1,3-bis(phenylmethyl)- synthesis
- Product Name:1H-Furo[3,4-d]imidazole-2,4-dione, tetrahydro-1,3-bis(phenylmethyl)-
- CAS Number:40222-71-7
- Molecular formula:C19H18N2O3
- Molecular Weight:322.36
![(cis)-1,3-dibenzyldihydro-1H-furo[3,4-d]imidazole-2,4,6(3H)-trione](/CAS/GIF/26339-42-4.gif)
26339-42-4
13 suppliers
inquiry
![1H-Furo[3,4-d]imidazole-2,4-dione, tetrahydro-1,3-bis(phenylmethyl)-](/CAS/20210111/GIF/40222-71-7.gif)
40222-71-7
1 suppliers
inquiry
Yield:40222-71-7 > 99 %Spectr.
Reaction Conditions:
with dichloro(benzene)ruthenium(II) dimer;potassium tert-butylate;hydrogen;4,5-bis(diphenylphosphino)-9,9-dimethylxanthene in toluene at 110; under 76005.1 Torr; for 12 h;Autoclave;
Steps:
4.3. General procedure for hydrogenation of 1
The procedure is represented with XANTPHOS as diphosphine ligand. [RuCl2(C6H6)]2 (1.0 mg, 2.0 μmol), XANTPHOS (2.3 mg, 4.0 μmol), and toluene (8.0 mL) were placed into a dry, argon-filled 20-mL Schlenk tube with a Young's tap. The solution was degassed three times by freeze-thaw method and was heated at 100 °C for 1 h. After the resulting pale yellow-colored solution was cooled to 25 °C, a 25 mM THF solution of t-C4H9OK (0.16 mL, 4.0 μmol), which has been degassed by three freeze-thaw cycles was added to the mixture. One-quarter (2 mL, 1.0 μmol) of the solution was transferred via stainless cannula to a pre-dried 50-mL stainless autoclave containing 1 (67.3 mg, 0.2 mmol) under argon pressure. Hydrogen was initially introduced under 10 atm pressure with several quick release-fill cycles before being pressurized to 100 atm. The solution was vigorously stirred for 12 h at 110 °C. After carefully venting the hydrogen gas, the resulting orange-colored homogeneous solution was concentrated under reduced pressure to give reddish oil of the crude product. The 1H NMR analysis (10:1 acetone-d6/acetonitrile-d3, 25 °C) showed that the conversion was 100% and the product ratio of lactone (2), hydroxy lactone (3), and dicarboxylic acid (4) was >99:<1:0. The yield was determined by 1H NMR analysis of a reaction mixture after addition of a 24 mg (0.2 mmol) of mesitylene. The area of the methyl signal of mesitylene (δ 2.29, factor 1.00) and the following signals of the compounds were compared: substrate 1, δ 4.63 (s, 2H, 2CH), factor 1.00; lactone 2, δ 4.20 (d, 1H, J=15.2 Hz, CHHC6H5), factor 1.00; dicarboxylic acid, δ 4.10 (d, 2H, J=20.5 Hz, CHHC6H5), factor 1.21; hydroxy lactone 3, δ 4.03 (d, 1H, J=15.0 Hz, CHHC6H5), factor 0.65. Physical properties of 2 and 3 were consistent with those previously reported. [5h] and [5i] Physical properties of 4 were consistent with those of the commercially available sample.
References:
Yoshimura, Masahiro;Tsuda, Kazuomi;Nakatsuka, Hiroshi;Yamamura, Tomoya;Kitamura, Masato [Tetrahedron,2011,vol. 67,# 51,p. 10006 - 10010] Location in patent:experimental part
![1H-Furo[3,4-d]imidazole-2,4-dione, tetrahydro-1,3-bis(phenylmethyl)-, (3aR,6aS)-](/CAS/20200611/GIF/87585-00-0.gif)
87585-00-0
1 suppliers
inquiry
![1H-Furo[3,4-d]imidazole-2,4-dione, tetrahydro-1,3-bis(phenylmethyl)-](/CAS/20210111/GIF/40222-71-7.gif)
40222-71-7
1 suppliers
inquiry