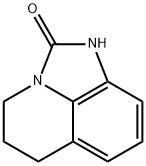
4H-Imidazo[4,5,1-ij]quinolin-2(1H)-one,5,6-dihydro-(7CI,9CI) synthesis
- Product Name:4H-Imidazo[4,5,1-ij]quinolin-2(1H)-one,5,6-dihydro-(7CI,9CI)
- CAS Number:4024-28-6
- Molecular formula:C10H10N2O
- Molecular Weight:174.2
Yield:4024-28-6 11.2 g (88%)
Reaction Conditions:
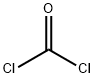
in ammonium hydroxide;acetic acid;chlorobenzene;
Steps:
2 5,6-Dihydro-4H-imidazo[4,5,1-ij]quinolin-2(1H)-one
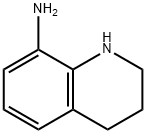
EXAMPLE 2 5,6-Dihydro-4H-imidazo[4,5,1-ij]quinolin-2(1H)-one To a solution of 10.77 g of 8-amino-1,2,3,4-tetrahydroquinoline in glacial acetic acid (30 ml) was added dropwise a 21.75% solution of phosgene in chlorobenzene (30 ml), under nitrogen, with stirring. After the addition was complete, the mixture was heated under reflux, with stirring for one hr and evaporated. The residue was taken up in 10% ammonium hydroxide solution (75 ml) and extracted with dichloromethane. The organic extracts were dried over anhydrous magnesium sulfate, filtered, and the filtrate was evaporated. Ether was added to the residue and the mixture was chilled. The precipitate was collected to give 11.2 g (88%) of product.
References:
US5500423,1996,A
54012-92-9
32 suppliers
inquiry
![4H-Imidazo[4,5,1-ij]quinolin-2(1H)-one,5,6-dihydro-(7CI,9CI)](/CAS/20180713/GIF/4024-28-6.gif)
4024-28-6
13 suppliers
$110.00/50mg

54012-92-9
32 suppliers
inquiry
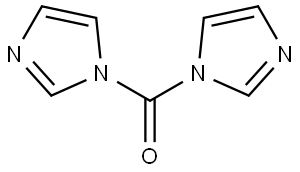
530-62-1
926 suppliers
$6.00/25g
![4H-Imidazo[4,5,1-ij]quinolin-2(1H)-one,5,6-dihydro-(7CI,9CI)](/CAS/20180713/GIF/4024-28-6.gif)
4024-28-6
13 suppliers
$110.00/50mg
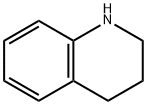
635-46-1
352 suppliers
$5.00/10g
![4H-Imidazo[4,5,1-ij]quinolin-2(1H)-one,5,6-dihydro-(7CI,9CI)](/CAS/20180713/GIF/4024-28-6.gif)
4024-28-6
13 suppliers
$110.00/50mg