
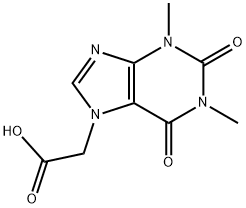
1,2,3,6-tetrahydro-1,3-dimethyl-2,6-dioxo-7H-purine-7-acetyl chloride synthesis
- Product Name:1,2,3,6-tetrahydro-1,3-dimethyl-2,6-dioxo-7H-purine-7-acetyl chloride
- CAS Number:40421-16-7
- Molecular formula:C9H9ClN4O3
- Molecular Weight:256.65
652-37-9
180 suppliers
$5.00/100mg

40421-16-7
5 suppliers
inquiry
Yield:-
Reaction Conditions:
with oxalyl dichloride;N,N-dimethyl-formamide in chloroform;acetonitrile at 20;
Steps:
12 N-[2-(3,4-Dichlorophenyl)-ethyl]-2-(1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydro-purin-7-yl)-acetamide (396)
A suspension of theophylline-7-acetic acid (2 mmol) in CHCl3 (15 mL) and MeCN (15 mL) was cooled in an ice-water bath. Oxalyl chloride (2.2 mmol) was then added dropwise. Catalytic DMF (25 μL) was then added. The mixture was stirred at room temperature over night. The solution was then cooled in an ice-water bath, and DMAP (2.5 mmol) was added in one portion. The substituted phenethylamine was added dropwise and the reaction mixture was stirred at room temperature over night. After diluting with CHCl3 (50 mL), the mixture washed with H2O, citric acid (10% in H2O), NaHCO3 (sat.), dried over Na2SO4 and concentrated in vacuo. The crude product was purified by flash chromatography on silica gel eluting with MeOH/EtOAc (18%).; N-[2-(3,4-Dichlorophenyl)-ethyl]-2-(1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydro-purin-7-yl)-acetamide (396) Compound 396 (490 mg, 56%) was prepared from 101 (506 mg, 2.1 mmol) and 106 (320 μL, 2.1 mmol) by General Procedure B. MS (APCI): m/z 410 [M+H]+.
References:
US2007/219222,2007,A1 Location in patent:Page/Page column 44