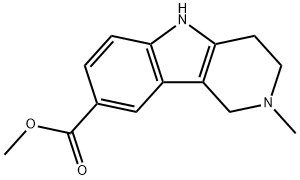
2-Methyl-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indole-8-carboxylic acid methyl ester synthesis
- Product Name:2-Methyl-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indole-8-carboxylic acid methyl ester
- CAS Number:404913-16-2
- Molecular formula:C14H16N2O2
- Molecular Weight:244.29

67-56-1
741 suppliers
$9.00/25ml

6296-89-5
49 suppliers
inquiry

34737-83-2
36 suppliers
inquiry
![2-Methyl-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indole-8-carboxylic acid methyl ester](/CAS/20180601/GIF/404913-16-2.gif)
404913-16-2
7 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: [4-(methoxycarbonyl)phenyl]hydrazine hydrochloride;1-methylpiperidin-4-one hydrochloridewith hydrogenchloride in water at 100;
Stage #2: methanolwith hydrogenchloride at 90;Reflux;
Stage #3: with sodium hydrogencarbonate in water;Product distribution / selectivity;
Steps:
12A
Example 12A [0413] Methyl-4- hydrazinylbenzoate hydrochloride (10 g, 50 mmol) and l-methylpiperidin-4- one hydrochloride (7.3g, 50 mmol) were dissolved in aqueous HCl and heated at 100 0C overnight (product was detected by LCMS). The reaction mixture was concentrated under vacuum and refluxed (90 0C) in methanolic HCl overnight (product was detected by LCMS & TLC). The reaction mixture was concentrated under vacuum and basified with aqueous NaHCO3 solution and extracted with EtOAc, dried over anhydrous sodium sulfate and concentrated under reduced pressure to obtain product. The product was crystallized in DCM and ether and hexane. Yield 5.1 g. 1U NMR (DMSO-J6, free base) d (ppm): 8.55 (s, IH), 8.27 (s, IH) 7.82-7.79 (d, IH), 7.24-7.20 (d, IH), 3.94 (s, 3H), 3.72 (s, 2H), 3.85 (s, 4H) 2.60 (s, 3H).
References:
WO2010/51501,2010,A1 Location in patent:Page/Page column 240