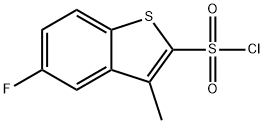
5-fluoro-2-chlorosulfonyl-3-Methylbenzo[b]thiophene synthesis
- Product Name:5-fluoro-2-chlorosulfonyl-3-Methylbenzo[b]thiophene
- CAS Number:404964-34-7
- Molecular formula:C9H6ClFO2S2
- Molecular Weight:264.72
![Benzo[b]thiophene-2-sulfonic acid, 5-fluoro-3-methyl-](/CAS/20210305/GIF/1353433-16-5.gif)
1353433-16-5
0 suppliers
inquiry
![5-fluoro-2-chlorosulfonyl-3-Methylbenzo[b]thiophene](/CAS/GIF/404964-34-7.gif)
404964-34-7
18 suppliers
$138.00/1g
Yield:404964-34-7 93%
Reaction Conditions:
with trichlorophosphate at 60;
Steps:
10
Intermediate 10-Fluoro-3-methyl-benzo[b]thiophene-2-sulfonyl chlorideA solution of commercially available 5-fluoro-3-methyl-l-benzothiophene (215 mg, 1.29 mmol), Ac20 (365 μ, 3.86 mmol) and cone. H2SO4 (83 μ) in EtOAc (4 mL) was shaken over night at room temp. The reaction mixture was diluted with EtOAc, washed with H20 and Brine and then dried. Evaporation of the solvent afforded 234 mg of 5-fluoro-3-methyl- benzo[b]thiophene-2-sulfonic acid (yield 74%). 1H NMR (400 MHz, DMSO-d6) δ ppm 2.45 (s, 3 H) 7.22 (td, 1 H) 7.52 (dd, 1 H) 7.89 (dd, 1 H).A mixture of 5-fluoro-3-methylbenzothiophene-2-sulfonic acid (230 mg, 0.93 mmol) in POCI3 (5 mL) was heated at 60 °C over night. The POCI3 was evaporated and the crude prod- uct was dissolved in CH2C12 and washed with water. The organic phase was dried and evaporated and gave 0.23 g (93%) of the title compound.
References:
WO2011/161201,2011,A1 Location in patent:Page/Page column 73
![5-FLUORO-3-METHYLBENZO[B]THIOPHENE](/CAS/GIF/17514-63-5.gif)
17514-63-5
79 suppliers
$15.00/250mg
![5-fluoro-2-chlorosulfonyl-3-Methylbenzo[b]thiophene](/CAS/GIF/404964-34-7.gif)
404964-34-7
18 suppliers
$138.00/1g
2968-13-0
47 suppliers
$26.39/1g
![5-fluoro-2-chlorosulfonyl-3-Methylbenzo[b]thiophene](/CAS/GIF/404964-34-7.gif)
404964-34-7
18 suppliers
$138.00/1g