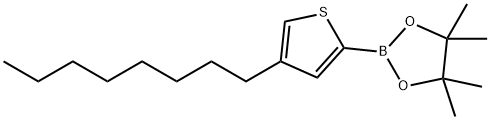
4-n-Octyl-2-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)thiophene synthesis
- Product Name:4-n-Octyl-2-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)thiophene
- CAS Number:405165-12-0
- Molecular formula:C18H31BO2S
- Molecular Weight:322.31
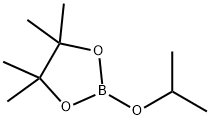
61676-62-8
312 suppliers
$11.19/5G
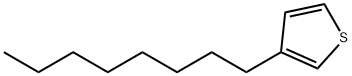
65016-62-8
202 suppliers
$6.00/1g

405165-12-0
34 suppliers
$45.00/50mg
Yield:405165-12-0 45%
Reaction Conditions:
Stage #1: 3-octylthiophenewith n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -78; for 2.25 h;Inert atmosphere;
Stage #2: 2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane in tert-Amyl alcohol;hexane at -78 - 25; for 19 h;
Steps:
1 Synthesis of 4,4,5, 5-tetramethyl-2-(4-octylthiophen-2-yl)-1 ,3,2-dioxaborolane having formula (4)
Synthesis of 4,4,5, 5-tetramethyl-2-(4-octylthiophen-2-yl)-1 ,3,2-dioxaborolane having formula (4) (0151) In a 50 ml three-neck flask, an inert atmosphere was created through nitrogen (N2) insufflation. Subsequently, 1.3 g (12.0 mmoles) of N ,N -di-i'so-propylamine (DIPA) anhydrous and 5 ml of tetrahydrofuran (THF) anhydrous were added. The solution obtained, kept under stirring, was cooled to 0°C and subsequently 4.8 ml of n-butyl- lithium (LiBu) (2.5 M in hexane) were added slowly, over 10 minutes. The reaction mixture obtained was kept, under stirring, at 0°C, for 30 minutes, subsequently, after cooling to -78°C, 2.0 g (10.0 mmoles) of 3-octylthiophene having formula (3) previously dissolved in 15 ml of tetrahydrofuran (THF) anhydrous were added slowly, over 15 minutes. The reaction mixture obtained was kept, under stirring, at -78°C, for 2 hours, at the end of which 2.3 g (12.00 mmol) of 2-i'so-propoxy-4,4,5,5-tetramethyl-1 ,3,2- dioxaborolane were added: the whole was kept under stirring, at -78°C, for another 2 hours. Subsequently, the reaction mixture was heated slowly, over 1 hour, to ambient temperature (25°C) and kept, at said temperature, under stirring, for 16 hours, at the end of which 30 ml of a 10% hydrochloric acid (HCI) solution were added. The residual solvent was removed through distillation at reduced pressure and the residue obtained was extracted with hexane (3 x 70 ml). The global organic phase (obtained by joining the three organic phases obtained from the extraction) was washed with a saturated aqueous solution of sodium chloride (NaCI) (2 x 70 ml) and subsequently anhydrified on sodium sulfate anhydrous for 3 hours. The residual solvent was removed by distillation at reduced pressure, obtaining a yellow oil. Said yellow oil was purified through (0152) chromatography on a silica gel column using a mixture of hexane/dichloromethane (8/2, v/v) as eluent, after pre-treating the silica with triethylamine (NEt3), obtaining 1.46 g (yield 45%) of 4,4,5,5-tetramethyl-2-(4-octylthiophen-2-yl)-1 ,3,2-dioxaborolane having formula (4), as colorless oil, which was characterized through 1 H-NMR (400 MHz, CDCI3) obtaining the following spectrum: δ 7.45 (s, 1 H), 7.19 (s, 1 H), 2.60 (t, J= 8.0 Hz, 2H), 1.59 (quintet, J= 8.0 Hz, 2H), 1.36 - 1.16 (m, 22H), 0.86 (t, J= 8.0 Hz, 3H) ppm.
References:
WO2017/199151,2017,A1 Location in patent:Page/Page column 29-30

65016-62-8
202 suppliers
$6.00/1g
25015-63-8
221 suppliers
$22.39/5G

405165-12-0
34 suppliers
$45.00/50mg