
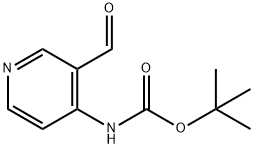
(3-HYDROXYMETHYL-PYRIDIN-4-YL)-CARBAMIC ACID TERT-BUTYL ESTER synthesis
- Product Name:(3-HYDROXYMETHYL-PYRIDIN-4-YL)-CARBAMIC ACID TERT-BUTYL ESTER
- CAS Number:407623-72-7
- Molecular formula:C11H16N2O3
- Molecular Weight:224.26
116026-93-8
83 suppliers
$70.00/250mg

407623-72-7
12 suppliers
inquiry
Yield:407623-72-7 63%
Reaction Conditions:
with sodium tetrahydroborate in methanol at 20; for 1 h;
Steps:
5.1.29. tert-Butyl [3-(hydroxymethyl)pyridin-4-yl]carbamate (15A)
tert-Butyl (3-formylpyridin-4-yl)carbamate12 M.C. Venuti, R.A. Stephenson, R. Alvarez, J.J. Bruno and A.M. Strosberg, J. Med. Chem. 31 (1988), p. 2136. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (60)12 was dissolved in MeOH (10 mL), and NaBH4 (205 mg, 5.43 mmol) was added. After the mixture was stirred at room temperature for 1 h, the solvent was distilled off in vacuo. The residue was partitioned between CH2Cl2 (50 mL) and brine (50 mL). Organic phase was dried over Na2SO4 and concentrated in vacuo. Obtained solid was washed with hexane to obtain the title compound (382 mg, 1.70 mmol, 63%) as a white solid. 1H NMR (CDCl3) δ: 1.53 (9H, s), 4.66 (2H, s), 7.93 (1H, s), 8.08 (1H, d, J = 5.9 Hz), 8.28 (1H, d, J = 5.9 Hz), 8.32 (1H, s). ESI-MS m/z: 225 (M+H)+.
References:
Mochizuki, Akiyoshi;Nagata, Tsutomu;Kanno, Hideyuki;Suzuki, Makoto;Ohta, Toshiharu [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 5,p. 1623 - 1642] Location in patent:experimental part

24424-99-5
824 suppliers
$13.50/25G

138116-34-4
107 suppliers
$26.00/100mg

407623-72-7
12 suppliers
inquiry

16135-36-7
182 suppliers
$9.00/1g

407623-72-7
12 suppliers
inquiry

7418-65-7
249 suppliers
$6.00/1g

407623-72-7
12 suppliers
inquiry