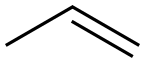
1,1'-Sulfonylbis[4-(prop-2-en-1-yloxy)benzene synthesis
- Product Name:1,1'-Sulfonylbis[4-(prop-2-en-1-yloxy)benzene
- CAS Number:41481-63-4
- Molecular formula:C18H18O4S
- Molecular Weight:330.4

Yield: 94%
Reaction Conditions:
Stage #1:4,4'-sulfonediphenol with potassium carbonate in acetone for 1 h;Inert atmosphere;Reflux;
Stage #2:allyl bromide in acetoneReflux;Inert atmosphere;
Steps:
D. General procedure for the allylation and Claisen rearrangement of bisphenol derivatives
General procedure: In a three-neck round-bottom flask the bisphenol derivative (1a-c) and potassium carbonate (0.9-1.5 equivalents per hydroxy group) were dissolved in acetone (approx. 10 wt% regarding the bisphenol component). After heating the white suspension to reflux for 60 minutes under a nitrogen atmosphere, allyl bromide 6 (1.3-1.5 equivalents per hydroxy group) was added through a dropping funnel. The mixture was refluxed until the reaction was completed (as monitored by TLC). Then deionized water was added and extracted three times with diethylether. The combined organic extracts were washed three times with 1N NaOH-solution and brine, dried over anhydrous MgSO4, filtered and concentrated under reduced pressure.
References:
Reinelt, Sebastian;Tabatabai, Monir;Fischer, Urs Karl;Moszner, Norbert;Utterodt, Andreas;Ritter, Helmut [Beilstein Journal of Organic Chemistry,2014,vol. 10,p. 1733 - 1740] Location in patent:supporting information