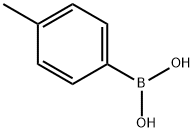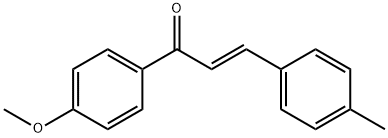
(2E)-1-(4-methoxyphenyl)-3-(4-methylphenyl)prop-2-en-1-one synthesis
- Product Name:(2E)-1-(4-methoxyphenyl)-3-(4-methylphenyl)prop-2-en-1-one
- CAS Number:41564-65-2
- Molecular formula:C17H16O2
- Molecular Weight:252.31
Yield:41564-65-2 99%
Reaction Conditions:
with sodium hydroxide in ethanol at 20; for 48 h;
Steps:
General procedure for chalcone synthesis (151-173).
General procedure: To a dry100 mL round-bottomed flask, semisynthetic acetophenone B or commercialacetophenone C or D (250 mg, between 1.22 and 2.08 mmol)and corresponding benzaldehyde (1.2 equiv.) were added, and thensolubilized in ethanol (5 mL). A NaOH 20% m/v solution (in 10 mL ofethanol) was added, and the mixture was stirred for 48 h. To stop the reaction, 5% HCl solution was added until pH ~ 7, and the mixture wasextracted with EtOAc (3x30 mL). The organic layer was dried withNa2SO4, filtered, and separated by column chromatography using ahexane/EtOAc, obtaining compounds 151-173 in yields between 27and 99%.
References:
Mellado, Marco;González, César;Mella, Jaime;Aguilar, Luis F.;Vi?a, Dolores;Uriarte, Eugenio;Cuellar, Mauricio;Matos, Maria J. [Bioorganic Chemistry,2021,vol. 108]
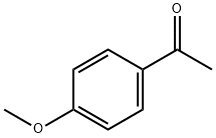
100-06-1
579 suppliers
$18.19/25g

41564-65-2
8 suppliers
inquiry