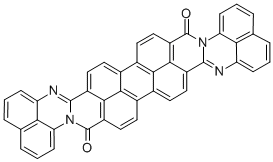
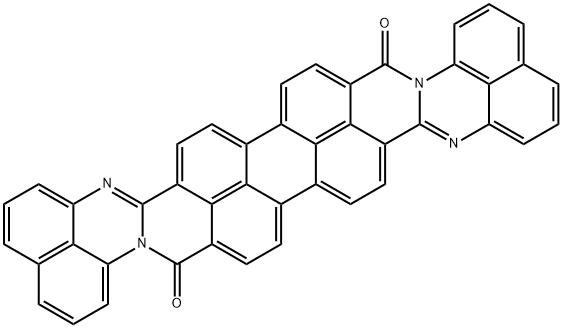
Anthra2",1",9":4,5,6:6",5",10":4',5',6'diisoquinolino2,1-a:2',1'-adiperimidine-8,20-dione(Mixturew\cisisomer) synthesis
- Product Name:Anthra2",1",9":4,5,6:6",5",10":4',5',6'diisoquinolino2,1-a:2',1'-adiperimidine-8,20-dione(Mixturew\cisisomer)
- CAS Number:41635-87-4
- Molecular formula:C44H20N4O2
- Molecular Weight:636.668

128-69-8
290 suppliers
$6.00/5g
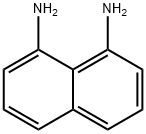
479-27-6
363 suppliers
$6.00/10g

41635-87-4
5 suppliers
inquiry
6859-32-1
12 suppliers
$498.64/5MG
Yield:-
Reaction Conditions:
with piperazine at 200; for 5 h;Overall yield = 98 %; Overall yield = 121 g;
Steps:
1
A Duplex kneader from Ika was charged with 78 g of perylenebisanhydride (198 mmol), 16 g of piperazine (186 mmol), 61 g of 1,8-diaminonaphthalene (386 mmol) and 55 g of tetraglycol, and this initial charge was kneaded while being heated to 200° C. over an hour and reacted at 200° C. for 4 hours. (0137) After cooling to room temperature, the kneaded material was introduced into 2% potash solution and stirred at 70° C. for two hours. (0138) The suspension was filtered off and washed in succession with water, 10% acetic acid and water. Drying at 80° C. left 121 g of black perylene pigment, corresponding to 98% of theory, in the form of a black powder.
References:
US8551237,2013,B2 Location in patent:Page/Page column 15