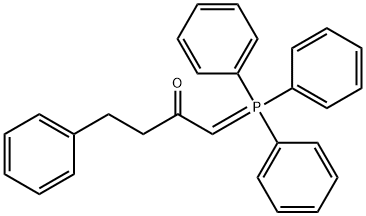
[1,1'-Biphenyl]-4-carboxylic acid (3aR,4R,5R,6aS)-hexahydro-2-oxo-4-[(1E)-3-oxo-5-phenyl-1-pentenyl]-2H-cyclopenta[b]furan-5-yl ester synthesis
- Product Name:[1,1'-Biphenyl]-4-carboxylic acid (3aR,4R,5R,6aS)-hexahydro-2-oxo-4-[(1E)-3-oxo-5-phenyl-1-pentenyl]-2H-cyclopenta[b]furan-5-yl ester
- CAS Number:41639-72-9
- Molecular formula:C31H28O5
- Molecular Weight:480.55
Yield:41639-72-9 91%
Reaction Conditions:
Stage #1: dimethyl(2-oxo-4-phenylbutyl)phosphonatewith potassium carbonate in isopropyl alcohol at 25; for 0.5 h;Horner-Wadsworth-Emmons Olefination;
Stage #2: (3aR,4R,5R,6aS)-4-formyl-2-oxohexahydro-2H-cyclopenta[b]furan-5-yl biphenyl-4-carboxylate in isopropyl alcohol at 25; for 5 h;Horner-Wadsworth-Emmons Olefination;Temperature;
Steps:
2 Example 2: Manufacture of (3aR,4R,5R,6aS)-2-oxo-4-((E)-3-oxo-5-phenylpent-1-enyl)hexahydro-2H-cyclopenta[b]furan-5-yl biphenyl-4-carboxylate.
Dimethyl 2-oxo-4-phenylbutylphosphonate (0.4 g, 1.43 mmol, 1 eq) was added to a 100 ml 3-neck flask and 10 ml of i-PrOH (IPA) was added and stirred. K2CO3 (0.6 g, 4.29 mmol, 3 eq) was added and stirred at 25 ° C for 30 minutes. Corey lactone aldehyde (0.5 g, 1.43 mmol, 1 eq) was added and stirred at 25 ° C for 3 hours. The reaction was warmed to 25 and stirred for 2 hours (TLC confirmed completion of the reaction, ethyl acetate (EA): hexane (Hx) = 1: 1, rf = 0.65). The reaction was concentrated under reduced pressure (bath 40 ° C). To the reaction were added 10 ml of water and 10 ml of methylene chloride (MC) and the layers were separated. The water layer was added with 10 ml of MC and extracted one more time. NaCl aqueous solution, and the mixture was dried over Na2SO4, filtered, concentrated under reduced pressure A solid compound was obtained.- 0.61g (yield: 91%). 1H NMR (400MHz, CDCl3): δ2.30 (d, J = 15.6 Hz, 2H), 2.51 (t, J = 11.8 Hz, 1H), 2.67 - 2.56 (m, 1H), 2.91 (dd , J = 18.3, 7.1 Hz, 6H), 5.10 (s, 1H), 5.31 (d, J = 4.9 Hz, 1H), 6.22 (d, J = 15.8 Hz, 1H, CH = CH), 6.66 (dd, J = 15.8, 7.6 Hz, 1H, CH = CH), 7.16 (dd, J = 14.6, 7.8 Hz, 3H, HAr), 7.16 (dd, J = 14.6, 7.8 Hz, 3H, HAr), 7.25 (t, J = 6.9 Hz, 2H, HAr), 7.43 (dt, J = 14.5, 7.6 Hz, 3H, HAr), 7.63 (dd, J = 19.0, 7.2 Hz, 4H, HAr), 8.09 - 8.01 (m, 2H, HAr).
References:
KR2016/15100,2016,A Location in patent:Paragraph 0078-0081
![[1,1'-Biphenyl]-4-carboxylic acid, (3aR,4R,5R,6aS)-hexahydro-4-[(1E,3R)-3-hydroxy-5-phenyl-1-penten-1-yl]-2-oxo-2H-cyclopenta[b]furan-5-yl ester](/CAS/20210305/GIF/41639-23-0.gif)
41639-23-0
0 suppliers
inquiry
![[1,1'-Biphenyl]-4-carboxylic acid (3aR,4R,5R,6aS)-hexahydro-2-oxo-4-[(1E)-3-oxo-5-phenyl-1-pentenyl]-2H-cyclopenta[b]furan-5-yl ester](/CAS2/GIF/41639-72-9.gif)
41639-72-9
58 suppliers
inquiry
![[1,1'-Biphenyl]-4-carboxylic acid, (3aR,4R,5R,6aS)-4-[1-(acetyloxy)-5-phenyl-2-pentyn-1-yl]hexahydro-2-oxo-2H-cyclopenta[b]furan-5-yl ester](/CAS/20210305/GIF/1251949-15-1.gif)
1251949-15-1
0 suppliers
inquiry
![[1,1'-Biphenyl]-4-carboxylic acid (3aR,4R,5R,6aS)-hexahydro-2-oxo-4-[(1E)-3-oxo-5-phenyl-1-pentenyl]-2H-cyclopenta[b]furan-5-yl ester](/CAS2/GIF/41639-72-9.gif)
41639-72-9
58 suppliers
inquiry
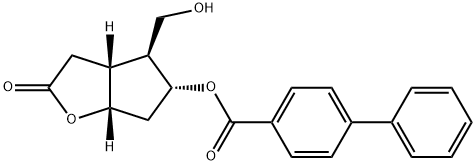
31752-99-5
245 suppliers
$19.00/250mg
![[1,1'-Biphenyl]-4-carboxylic acid (3aR,4R,5R,6aS)-hexahydro-2-oxo-4-[(1E)-3-oxo-5-phenyl-1-pentenyl]-2H-cyclopenta[b]furan-5-yl ester](/CAS2/GIF/41639-72-9.gif)
41639-72-9
58 suppliers
inquiry
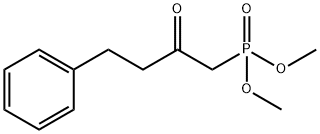
![[1,1'-Biphenyl]-4-carboxylic acid (3aR,4R,5R,6aS)-4-formylhexahydro-2-oxo-2H-cyclopenta[b]furan-5-yl ester](/CAS2/GIF/38754-71-1.gif)