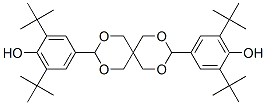
4,4'-(2,4,8,10-tetraoxaspiro[5.5]undecane-3,9-diyl)bis[2,6-di-tert-butylphenol] synthesis
- Product Name:4,4'-(2,4,8,10-tetraoxaspiro[5.5]undecane-3,9-diyl)bis[2,6-di-tert-butylphenol]
- CAS Number:41715-24-6
- Molecular formula:C35H52O6
- Molecular Weight:568.7838
Yield:41715-24-6 75%
Reaction Conditions:
with boron trifluoride diethyl etherate in tetrahydrofuran at 20; for 6 h;Inert atmosphere;Cooling with ice;Reagent/catalyst;Solvent;Time;Temperature;
Steps:
2; 2 (Example 2) Production of a compound represented by the formula (I-2)
Under a nitrogen atmosphere, 20.0 g of 1/2 hydrate of the compound represented by the formula (I-2-1), 5.6 g of the compound represented by the formula (I-2-2), and 200 mL of tetrahydrofuran are placed in the reaction vessel. Was added.While cooling with ice, 35.0 g of boron trifluoride-ethyl ether complex was added dropwise, and the mixture was stirred at room temperature for 6 hours.The reaction solution was poured into saturated aqueous sodium hydrogen carbonate solution and extracted with toluene.The organic layer was washed successively with water and saline. By performing column chromatography (silica gel, dichloromethane / hexane) and recrystallization (acetone / acetonitrile), 17.5 g of the compound represented by the formula (I-2) was obtained (yield 75%).No impurities were detected with the tert-butyl group eliminated.
References:
JP2020/158423,2020,A Location in patent:Paragraph 0211-0212; 0245-0246

