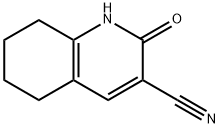
2-OXO-1,2,5,6,7,8-HEXAHYDRO-3-QUINOLINECARBONITRILE synthesis
- Product Name:2-OXO-1,2,5,6,7,8-HEXAHYDRO-3-QUINOLINECARBONITRILE
- CAS Number:4241-13-8
- Molecular formula:C10H10N2O
- Molecular Weight:174.2
Yield: 48%
Reaction Conditions:
Stage #1:cyclohexanone at 20; for 24 h;Inert atmosphere;
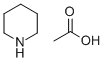
Stage #2:cyanoacetic acid amide with piperdinium acetate in waterInert atmosphere;Reflux;
Steps:
2-Oxo-1,2,5,6,7,8-hexahydroquinoline-3-carbonitrile 7
A mixture of cyclohexanone 8 (10.0 mL, 96.0 mmol) and m ethyl formate (5.95 mL, 96.0mmol) was added dropwise over 30 min to a solution of sodium methoxide which wasprepared by adding sodium (2.80 g, 0.120 mol) to dry methanol (22 mL). The reactionmixture was stirred at r.t. overnight and then diluted with ether and filtered to give thedesired product (9.5 g, 66%) as a cream solid.The salt (6.0 g, 0.042 mol) was dissolved in H2O (33 mL), followed by addition ofcyanoacetamide (3.9 g, 0.046 mol), and freshly prepared piperidinium acetate solution (2.9mL). (The piperidinium acetate solution was prepared by mixing acetic acid (4.20 mL),water (10 mL), and piperidine (7.20 mL)). The mixture was heated at reflux overnightbefore being acidified with acetic acid (4.5 mL). The reaction mixture was allowed tocool to r.t. and stirred for a further 12 h before the residue was filtered off, washed with icewater and collected to give the title compound 7 (3.47 g, 48%) as a white solid which wasused in the next reaction without further purification. m.p. > 230 °C. (Lit. 245-248 °C.5) δH(400 MHz, (CD3)2SO) 1.62-1.69 (4H, m, H-6 and H-7), 2.41 (2H, t, J = 5.7 Hz, H-5), 2.55(2H, t, J = 5.7 Hz, H-8), 7.88 (1H, s, H-4), 12.27 (1H, br s, NH). The 1H NMR data was inagreement with the literature values.6
References:
Pilkington, Lisa I.;Haverkate, Natalie A.;Van Rensburg, Michelle;Reynisson, Johannes;Leung, Euphemia;Barker, David [Synlett,2016,vol. 27,# 20,art. no. ST-2016-D0513-L,p. 2811 - 2814] Location in patent:supporting information

108-94-1
527 suppliers
$12.00/50g
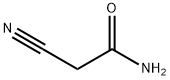
107-91-5
541 suppliers
$5.00/10g
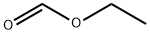
109-94-4
462 suppliers
$10.00/10g

4241-13-8
22 suppliers
inquiry

108-94-1
527 suppliers
$12.00/50g

4241-13-8
22 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)