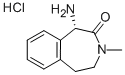
2H-3-Benzazepin-2-one, 1-amino-1,3,4,5-tetrahydro-3-methyl-, hydrochloride (1:1), (1S)- synthesis
- Product Name:2H-3-Benzazepin-2-one, 1-amino-1,3,4,5-tetrahydro-3-methyl-, hydrochloride (1:1), (1S)-
- CAS Number:425663-71-4
- Molecular formula:C11H14N2O.ClH
- Molecular Weight:226.706
![1-aMino-3-Methyl-4,5-dihydro-1H-benzo[d]azepin-2(3H)-one](/CAS/GIF/253324-91-3.gif)
253324-91-3
16 suppliers
$65.00/5mg

425663-71-4
32 suppliers
inquiry
Yield:425663-71-4 78%
Reaction Conditions:
Stage #1: 1-amino-3-methyl-1H,4H,5H-benzo-[d]azaperhydroepin-2-onewith (R)-Mandelic Acid in Isopropyl acetate;isopropyl alcohol at 45; for 3 h;
Stage #2: with 5-Nitrosalicylaldehyde in Isopropyl acetate;isopropyl alcohol; for 13 h;
Stage #3: with hydrogenchloride;water in ethyl acetate at 50; for 3 h;
Steps:
A5
[Example A5]; Preparation of (S)-1-amino-3-methyl-1,3,4,5-tetrahydrobenz[d]azepin-2-one hydrochloride; 36.8 g (242 mmol) of D-mandelic acid and 254 ml of 2-propanol were added into a flask, and were dissolved at 45°C. To this solution, an isopropyl acetate solution (169 ml) containing 47.0 g (247 mmol) of 1-amino-3-methyl-1,3,4,5-tetrahydrobenz[d]azepin-2-one obtained by the method of Example A4 was added dropwise at 45°C, followed by stirring for three hours. The reaction mixture was added with 2.06 g (12.4 mmol) of 5-nitrosalicylaldehyde, followed by further stirring for 13 hours. The resultant mixture was cooled down to a room temperature. Then, resulting crystals were filtered off, and suspended in 423 ml of ethyl acetate, to which 34.3 ml of concentrated hydrochloric acid was added at 50°C, followed by stirring for three hours. The resultant mixture was cooled down to a room temperature. Then, crystals were filtered off, washed, and dried to obtain 43.6 g (192 mmol, yield 78%, 99%ee) of (S)-1-amino-3-methyl-1,3,4,5-tetrahydrobenz[d]azepin-2-one hydrochloride as white crystals. 1H-NMR (400 MHz, DMSO-d6) δ 2.96 (3H, s), 3.24-3.34 (1H, m), 3.42-3.55 (2H, m), 4.30-4.39 (1H, m), 5.98 (1H, s), 7.39-7.50 (4H, m), 9.01 (3H, s).
References:
EP1985615,2008,A1 Location in patent:Page/Page column 18

13230-84-7
2 suppliers
inquiry

425663-71-4
32 suppliers
inquiry
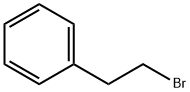
103-63-9
494 suppliers
$9.00/10g

425663-71-4
32 suppliers
inquiry
![3-Methyl-1,3,4,5-tetrahydrobenzo[d]azepin-2-one](/CAS/GIF/73644-95-8.gif)
73644-95-8
28 suppliers
inquiry

425663-71-4
32 suppliers
inquiry
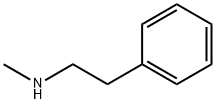
589-08-2
127 suppliers
$31.80/1gm:

425663-71-4
32 suppliers
inquiry