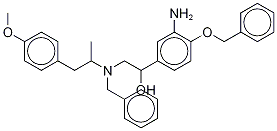
RAC-N-BENZYL-N-[2-HYDROXYL-2-(4-BENZYLOXY-3-AMINOPHENYL)-ETHYL]-3-(4-METHOXYPHENYL)-2-PROPYLAMINE-D6 synthesis
- Product Name:RAC-N-BENZYL-N-[2-HYDROXYL-2-(4-BENZYLOXY-3-AMINOPHENYL)-ETHYL]-3-(4-METHOXYPHENYL)-2-PROPYLAMINE-D6
- CAS Number:43229-68-1
- Molecular formula:C32H36N2O3
- Molecular Weight:496.64
aMino]Methyl]-3-nitro-4-(phenylMethoxy)benzeneMethanol](/CAS/20180808/GIF/43229-67-0.gif)
43229-67-0
19 suppliers
inquiry
![RAC-N-BENZYL-N-[2-HYDROXYL-2-(4-BENZYLOXY-3-AMINOPHENYL)-ETHYL]-3-(4-METHOXYPHENYL)-2-PROPYLAMINE-D6](/CAS/20140608/GIF/CB1507768.gif)
43229-68-1
22 suppliers
inquiry
Yield:43229-68-1 296 g
Reaction Conditions:
with iron;ammonium chloride in ethanol;water at 75 - 85;
Steps:
1.2
2) A compound of the formula IV (220 mL) and absolute ethanol (1280 mL) dissolved in absolute ethanol were added to the reaction flask and stirred.Reduced iron powder (199.7 g) was added, and the residue was washed with absolute ethanol (1000 mL), then ammonium chloride (191.3 g) and water (440 mL) were added.Heat to an internal temperature of 75-85 ° C, reaction 2-3h to complete (HPLC detection, peak area according to normalization method,If the compound of formula IV is less than 5.0%, the reaction is considered complete, then the temperature is lowered to 75±1 ° C, ethyl acetate is added, and the mixture is cooled to 20-30 ° C with stirring.After washing with ethyl acetate (550 mL), filtering, combining all the filtrates, the filtrate was obtained at 50-60 ° C,The organic layer and ethanol were evaporated under reduced pressure, and ethyl acetate (1650 mL) and water (1100 mL) were added and stirred, and the aqueous layer was removed by liquid.The organic phase was washed once with 10% aqueous sodium carbonate (1100 mL) and then once with 20% aqueous sodium chloride (1100 mL).Anhydrous magnesium sulfate (220 g) and activated carbon (22 g) were stirred for 1-2 h.After drying, the desiccant was removed by filtration and washed three times with ethyl acetate (220 mL) at a temperature of 50-60 ° C.It was concentrated to dryness under reduced pressure to give a red-brown viscous compound 296 g. Purity: 57.92%, diastereomer: 40.65%.
References:
CN109535027,2019,A Location in patent:Paragraph 0091; 0094; 0095; 0122; 0124
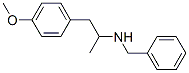
43229-65-8
160 suppliers
$74.00/250mg
![RAC-N-BENZYL-N-[2-HYDROXYL-2-(4-BENZYLOXY-3-AMINOPHENYL)-ETHYL]-3-(4-METHOXYPHENYL)-2-PROPYLAMINE-D6](/CAS/20140608/GIF/CB1507768.gif)
43229-68-1
22 suppliers
inquiry
![[3-Nitro-4-(phenylmethoxy)phenyl]-oxirane](/CAS/GIF/51582-41-3.gif)
51582-41-3
68 suppliers
inquiry
![RAC-N-BENZYL-N-[2-HYDROXYL-2-(4-BENZYLOXY-3-AMINOPHENYL)-ETHYL]-3-(4-METHOXYPHENYL)-2-PROPYLAMINE-D6](/CAS/20140608/GIF/CB1507768.gif)
43229-68-1
22 suppliers
inquiry