
(2-AMINOMETHYL-BENZYL)-CARBAMIC ACID TERT-BUTYL ESTER synthesis
- Product Name:(2-AMINOMETHYL-BENZYL)-CARBAMIC ACID TERT-BUTYL ESTER
- CAS Number:439116-13-9
- Molecular formula:C13H20N2O2
- Molecular Weight:236.31
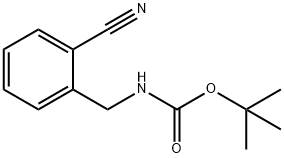
439118-51-1
23 suppliers
inquiry

439116-13-9
19 suppliers
$109.00/50mg
Yield: 43%
Reaction Conditions:
with sodium tetrahydroborate;water;cobalt(II) chloride in tetrahydrofuran at 35; for 12 h;
Steps:
tert-Butyl N-[[2-(aminomethyl)phenyl]methyl]carbamate 38.4
To a solution of compound 38.3 (4.00 g, 17.2 mmol) in tetrahydrofuran (40 mL) was added the solution of cobaltous chloride (4.10 g, 17.2 mmol) in water (20 mL). Then sodiumborohydride (1.30 g, 34.4 mmol) was added, the mixture stirred at 35°C for 12 h, diluted with water (20 mL), and extracted with ethyl acetate (3 x 50 mL). The combined organic phases were washed with brine (3 x 50 mL) and dried with anhydrous sodium sulfate. After filtration and concentration, the residue was purified by reverse phase flash chromatography (ammonium hydroxide conditions) to give compound 38.4 (1.70 g, 43% yield) as yellow oil.1H NMR (CD3OD, 400 MHz) O 1.45 (5, 9H), 3.94 (5, 2H), 4.37 (d, 2H), 6.00 (br. 5, 1H), 7.24- 7.26 (m, 1 H), 7.28 - 7.32 (m, 2H), 7.34- 7.36 (m, 1 H).
References:
THE UNIVERSITY OF SHEFFIELD;RICHARDS, Gareth;SKERRY, Timothy, M.;HARRITY, Joseph, P.A.;ZIRIMWABAGABO, Jean-Olivier;TOZER, Matthew, J.;GIBSON, Karl, Richard;PORTER, Roderick, Alan;BLANEY, Paul, Matthew;GLOSSOP, Paul, Alan WO2018/211275, 2018, A1 Location in patent:Page/Page column 297; 298
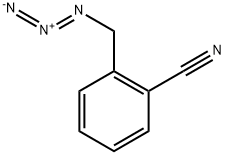
40508-03-0
10 suppliers
inquiry

439116-13-9
19 suppliers
$109.00/50mg
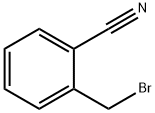
22115-41-9
441 suppliers
$5.00/5g

439116-13-9
19 suppliers
$109.00/50mg

24424-99-5
820 suppliers
$13.50/25G

439116-13-9
19 suppliers
$109.00/50mg

344957-25-1
61 suppliers
inquiry

439116-13-9
19 suppliers
$109.00/50mg