
1-[4-(4-METHOXYPHENYL)TETRAHYDRO-2H-PYRAN-4-YL]METHANAMINE synthesis
- Product Name:1-[4-(4-METHOXYPHENYL)TETRAHYDRO-2H-PYRAN-4-YL]METHANAMINE
- CAS Number:440087-51-4
- Molecular formula:C13H19NO2
- Molecular Weight:221.3
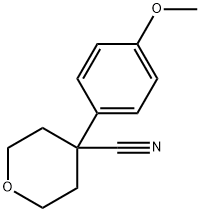
3648-78-0
29 suppliers
$72.00/100mg
![1-[4-(4-METHOXYPHENYL)TETRAHYDRO-2H-PYRAN-4-YL]METHANAMINE](/CAS/GIF/440087-51-4.gif)
440087-51-4
30 suppliers
inquiry
Yield:440087-51-4 100%
Reaction Conditions:
Stage #1: 4-(4-methoxyphenyl)tetrahydro-2H-pyran-4-carbonitrilewith lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20; for 0.416667 h;
Stage #2: with sodium hydroxide;water in tetrahydrofuran;
Steps:
3.1
Intermediate 3: 4-(4-Dimethylaminomethyl-tetrahydro-pyran-4-yl)-phenol [] Step 1:; To a stirred suspension of LiAlH4 (79g, 2.08mol, 5eq) in THF (900ml) at 0 to 5°C under an atmosphere of nitrogen was added 4-(4-methoxyphenyl)- tetrahydro-2H-pyran-4-carbonitrile (90g, 0.414mol) in THF (900ml) over a 25 minutes maintaining the temperature at 5 to 10°C. The reaction mixture was allowed to warm to ambient temperature and stirred until complete. Sodium hydroxide (2N, 850ml) was added dropwise, the resulting solids filtered and washed with THF (2x800ml), the organics concentrated in vacuo at 40°C. The residue was dissolved in EtOAc (300ml) and dried over MgSO4, filtered and concentrated in vacuo at 40°C to provide {[4-(4-methoxyphenyl)- tetrahydro-2H-pyran-4-yl]methyl}amine as a light yellow oil (92g, quantitative).1H NMR (400MHz, CDCl3) δ7.21 (d, 2H), 6.92 (d, 2H), 3.82 (s, 3H), 3.85-3.74 (m, 2H), 3.59-3.49 (m, 2H), 2.76 (s, 2H), 2.17-2.07 (m, 2H), 1.81 (ddd, 2H).
References:
EP1593679,2005,A1 Location in patent:Page/Page column 24-25
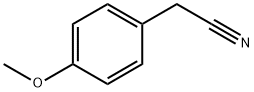
104-47-2
456 suppliers
$6.00/10g
![1-[4-(4-METHOXYPHENYL)TETRAHYDRO-2H-PYRAN-4-YL]METHANAMINE](/CAS/GIF/440087-51-4.gif)
440087-51-4
30 suppliers
inquiry