Methyl 1-[(4-methoxyphenyl)methyl]-1H-indazole-3-carboxylate synthesis
- Product Name:Methyl 1-[(4-methoxyphenyl)methyl]-1H-indazole-3-carboxylate
- CAS Number:441717-61-9
- Molecular formula:C17H16N2O3
- Molecular Weight:296.32
Yield:-
Reaction Conditions:
Stage #1: methyl 1H-indazole-3-carboxylatewith caesium carbonate in N,N-dimethyl-formamide at 0; for 0.166667 h;
Stage #2: p-methoxybenzyl chloride in N,N-dimethyl-formamide at 0 - 20; for 1 h;Inert atmosphere;
Steps:
1-6-1 Preparation of methyl 1 -(4-methoxybenzyl)-1 /-/-indazole-3-carboxylate
20.2 g of methyl 1 /-/-indazole-3-carboxylate (1 14 mmol) were dissolved in 123 mL of dry DMF and cooled to 0 °C. 59.7 g of cesium carbonate (183.1 mmol, 1 .6 eq.) were added and stirred for 10 min. 23.3 g of 1 -(chloromethyl)-4-methoxybenzene (148 mmol, 1 .3 eq.) were added dropwise at 0 °C. The mixture was stirred at room temperature for 1 hour under nitrogen atmosphere. Then the reaction mixture was partitioned between water and ethyl acetate. The organic layer was dried over sili- con filter and concentrated in vacuo. The residue was purified by flash chromatography to yield 20.9 g (60 mmol, 52 %) of 85% pure target compound.
References:
WO2014/147204,2014,A1 Location in patent:Page/Page column 101
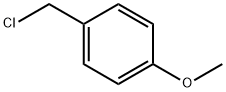
2393-23-9
460 suppliers
$20.00/25g

43120-28-1
153 suppliers
$10.00/1g
![Methyl 1-[(4-methoxyphenyl)methyl]-1H-indazole-3-carboxylate](/CAS/GIF/441717-61-9.gif)
441717-61-9
6 suppliers
inquiry
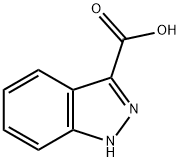
4498-67-3
464 suppliers
$6.00/5g
![Methyl 1-[(4-methoxyphenyl)methyl]-1H-indazole-3-carboxylate](/CAS/GIF/441717-61-9.gif)
441717-61-9
6 suppliers
inquiry