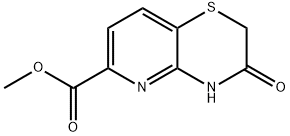
Methyl3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylate synthesis
- Product Name:Methyl3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylate
- CAS Number:443956-13-6
- Molecular formula:C9H8N2O3S
- Molecular Weight:224.24
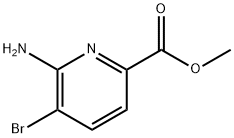
178876-82-9
107 suppliers
$34.00/100mg
![Methyl3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylate](/CAS/20180808/GIF/443956-13-6.gif)
443956-13-6
10 suppliers
inquiry
Yield:443956-13-6 100%
Reaction Conditions:
Stage #1: ethyl 2-sulfanylacetatewith sodium hydride in N,N-dimethyl-formamide at 0; for 1 h;
Stage #2: 6-amino-5-bromo-pyridine-2-carboxylic acid methyl ester in N,N-dimethyl-formamide at 20; for 16 h;
Steps:
2.a
A solution of ethyl 2-mercaptoacetate (1.473 mL) in DMF (48 ml_) was ice-cooled and treated with sodium hydride (540 mg of a 60% dispersion in oil). After 1 h methyl 6- amino-5-bromopyridine-2-carboxylate (3 g) (T.R. Kelly and F. Lang, J. Org. Chem. 61, 1996, 4623-4633) was added and the mixture stirred for 16h at room temperature. The solution was diluted with EtOAc (1 L), washed with water (3 x 300 mL), dried and evaporated to about 10 mL. The white solid was filtered off and washed with a little EtOAc to give the ester (0.95g); LC/MS (APCI") m/z 223 ([M-H]", 100%).
References:
WO2006/81178,2006,A2 Location in patent:Page/Page column 20-21

623-51-8
228 suppliers
$7.00/25g

178876-82-9
107 suppliers
$34.00/100mg
![Methyl3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylate](/CAS/20180808/GIF/443956-13-6.gif)
443956-13-6
10 suppliers
inquiry

2365-48-2
341 suppliers
$14.00/25g

178876-82-9
107 suppliers
$34.00/100mg
![Methyl3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylate](/CAS/20180808/GIF/443956-13-6.gif)
443956-13-6
10 suppliers
inquiry