
3-((4-((4-butylphenyl)(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)amino)phenyl)(methyl)bo synthesis
- Product Name:3-((4-((4-butylphenyl)(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)amino)phenyl)(methyl)bo
- CAS Number:444289-55-8
- Molecular formula:C34H45B2NO4
- Molecular Weight:553.35
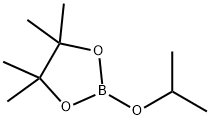
61676-62-8
321 suppliers
$11.19/5G
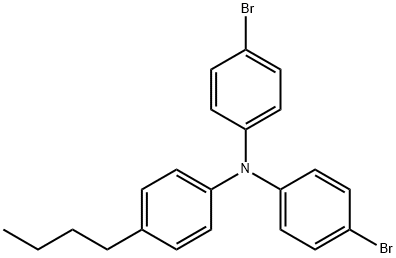
276690-04-1
13 suppliers
inquiry

444289-55-8
14 suppliers
inquiry
Yield:444289-55-8 53%
Reaction Conditions:
Stage #1: 4-bromo-N-(4-bromophenyl)-N-(4-butylphenyl)anilinewith n-butyllithium in tetrahydrofuran;hexane at -78; for 1 h;
Stage #2: 2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane in tetrahydrofuran;hexane at -78; for 2 h;
Steps:
1
Compound 3 was prepared by the same procedure as in the reported process (Chu YL, Cheng CC, Yen YC, Chang FC, A new supramolecular hole injection / transport material on conducting polymers for application in light-emitting diodes. Advanced materials 2012; 24: 1894-8) .A solution of 4-bromo-N- (4-bromophenyl) -N- (4-butylphenyl) aniline (1.97 g, 4.3 mmol) in dry THF (45 mL) was cooled to- .nBuLi (2.5 M / hexane, 4.3 mL, 10.7 mmol) was added dropwise and the mixture was stirred at the same temperature for 1 h.Next, 2-isopropyl-4,4,5,5-tetraethyl-1,3,2-dioxaborolane (2-isopropoxy-4,4,5,5-tetramethyl- -dioxaborolane, 2.7 mL, 12.8 mmol) was added and the mixture was stirred at -78 [deg.] C for 2 h. The reaction was quenched with the addition of water, extracted with ethyl acetate, and the organic layer was dried with sodium sulfate. The solvent was removed and the crude product was quenched by recrystallization from methanol to give a white solid (1.26 g, 53%).
References:
KR2018/17709,2018,A Location in patent:Paragraph 0157; 0160; 0169-0171
152270-84-3
2 suppliers
inquiry

444289-55-8
14 suppliers
inquiry

276690-04-1
13 suppliers
inquiry

444289-55-8
14 suppliers
inquiry
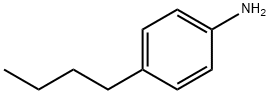
104-13-2
218 suppliers
$7.00/5g

444289-55-8
14 suppliers
inquiry

108-86-1
510 suppliers
$10.00/5g

444289-55-8
14 suppliers
inquiry