
4,7-Bis(5-broMo-4-hexylthiophen-2-yl)benzo[c][1,2,5]thiadiazole synthesis
- Product Name:4,7-Bis(5-broMo-4-hexylthiophen-2-yl)benzo[c][1,2,5]thiadiazole
- CAS Number:444579-39-9
- Molecular formula:C26H30Br2N2S3
- Molecular Weight:626.53
Yield: 50% , 30%
Reaction Conditions:
with N-Bromosuccinimide in chloroform at 0 - 20; for 2.5 h;Darkness;Inert atmosphere;Schlenk technique;
Steps:
2.1 2.2.1. Synthesis of DTBT-Br and DTBT-2Br
A solution of compound 1 (4.68 g, 10 mmol) dissolved in 150 mL of chloroform was cooled to 0 °C, then N-bromosuccinimide (1.76 g, 10 mmol) was added in portions over a course of 30 min without light, and the resulted mixture was stirred for another 2 h at room temperature. Then 100 mL of water was added to quench the reaction. The mixture was extracted with chloroform; the resulting organic phase was washed with brine and dried over anhydrous MgSO4. The solvent was removed under reduced pressure and the residue was purified by column chromatography on silica gel using a mixture solvent of petroleum ether (PE) and CH2Cl2 (5:1, v:v) as eluent to give DTBT-Br (yield: 50%) and DTBT-2Br (yield: 30%) as an orange solid.
References:
Deng, Jiyong;Chen, Jianhua;Tao, Qiang;Yan, Dong;Fu, Yafen;Tan, Hua [Tetrahedron,2018,vol. 74,# 29,p. 3989 - 3995]
![4,7-Bis(4-hexylthiophen-2-yl)benzo[c][1,2,5]thiadiazole](/CAS/20180703/GIF/761416-46-0.gif)
761416-46-0
19 suppliers
inquiry
![4,7-Bis(5-broMo-4-hexylthiophen-2-yl)benzo[c][1,2,5]thiadiazole](/CAS/20150408/GIF/444579-39-9.gif)
444579-39-9
46 suppliers
inquiry
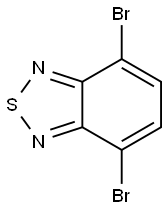
15155-41-6
321 suppliers
$20.00/1g
![4,7-Bis(5-broMo-4-hexylthiophen-2-yl)benzo[c][1,2,5]thiadiazole](/CAS/20150408/GIF/444579-39-9.gif)
444579-39-9
46 suppliers
inquiry
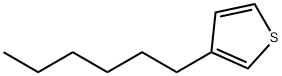
1693-86-3
274 suppliers
$10.00/1g
![4,7-Bis(5-broMo-4-hexylthiophen-2-yl)benzo[c][1,2,5]thiadiazole](/CAS/20150408/GIF/444579-39-9.gif)
444579-39-9
46 suppliers
inquiry
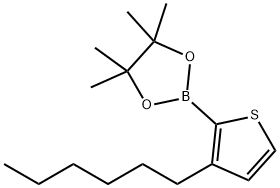
850881-09-3
69 suppliers
$60.00/1g

15155-41-6
321 suppliers
$20.00/1g
![4,7-Bis(5-broMo-4-hexylthiophen-2-yl)benzo[c][1,2,5]thiadiazole](/CAS/20150408/GIF/444579-39-9.gif)
444579-39-9
46 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)