
{2-[4-(trifluoroMethyl)phenyl]-1,3-thiazole-4,5-diyl}diMethanol synthesis
- Product Name:{2-[4-(trifluoroMethyl)phenyl]-1,3-thiazole-4,5-diyl}diMethanol
- CAS Number:444615-64-9
- Molecular formula:C12H10F3NO2S
- Molecular Weight:289.27
Yield:444615-64-9 77.4%
Reaction Conditions:
with sodium tetrahydroborate;acetic acid in tetrahydrofuran at 45 - 50; for 3 h;
Steps:
1 Example 1; {5-(hydroxymethyl)-2-[4-(trifluoromethyl)phenyl]-1,3-thiazol-4-yl}methanol
Diethyl 2-[4-(trifluoromethyl)phenyl]-1,3-thiazole-4,5-dicarboxylate (200 g, 0.536 moles), methanol (142.5 g, 4.45 moles, 0.71 wt., 8.3 equiv.) and acetic acid (0.64 g, 0.01 moles, 0.0032 wt., 0.019 equiv.) were dissolved in tetrahydrofuran (530 ml, 2.65 volumes). This solution was added drop-wise to a stirred slurry of sodium borohydride (84.1 g, 2.22 moles, 0.42 wt., 4.1 equiv.) in tetrahydrofuran (1330 ml, 6.65 volumes) over about a 45 minute period. During the addition, there gas evolution (H2) and a 28 C temperature rise. On completion of the addition, the reaction mixture was held at 45-50 C for about 3 hours. Toluene (1330 ml, 6.65 volumes) was added to the cooled reaction mixture (20 C), and the reaction mixture was quenched with aqueous 2N hydrochloric acid (1600 ml, 8 volumes). The layers were separated and the organic layer was concentrated (40 C, vacuum) to about one-half the original volume. The concentrated organic layer was then treated with hexanes (334 ml, 1.67 volumes) with stirring, and the product crystallized from solution, was filtered and washed with toluene/hexanes 1:1 (2x200 ml, 2x1 volume) and then dried in vacuo at 45 C. Yield: 120 grams, 77.4 % of theory. 1H NMR (300 MHz, CD3OD): δ 8.12 (2H; d, J=8.3Hz), 7.85 (2H, d, J=8.3 Hz), 6.54 (1H, br s), 5.72 (1H, br s), 4.77 (2H, s), 4.79 (2H, s), 4.58 (2H, s), 4.77 (2H, s)
References:
WO2004/26849,2004,A1 Location in patent:Page 8; 9
![diethyl 2-[4-(trifluoroMethyl)phenyl]-1,3-thiazole-4,5-dicarboxylate](/CAS/20150408/GIF/444615-63-8.gif)
444615-63-8
7 suppliers
inquiry
![{2-[4-(trifluoroMethyl)phenyl]-1,3-thiazole-4,5-diyl}diMethanol](/CAS/20150408/GIF/444615-64-9.gif)
444615-64-9
6 suppliers
inquiry
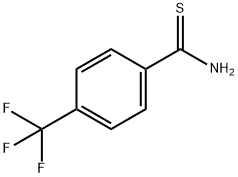
72505-21-6
164 suppliers
$10.00/5g
![{2-[4-(trifluoroMethyl)phenyl]-1,3-thiazole-4,5-diyl}diMethanol](/CAS/20150408/GIF/444615-64-9.gif)
444615-64-9
6 suppliers
inquiry
