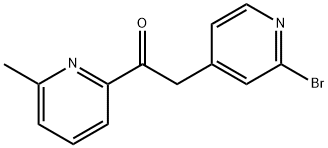
2-(2-BROMOPYRIDIN-4-YL)-1-(6-METHYLPYRIDIN-2-YL)ETHANONE synthesis
- Product Name:2-(2-BROMOPYRIDIN-4-YL)-1-(6-METHYLPYRIDIN-2-YL)ETHANONE
- CAS Number:446880-80-4
- Molecular formula:C13H11BrN2O
- Molecular Weight:291.14
Yield:446880-80-4 70%
Reaction Conditions:
with sodium hexamethyldisilazane in tetrahydrofuran at -30 - 20;
Steps:
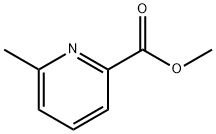
1 Intermediate 1:2-[2-bromo-pyridin-4-yl]-1- (6-methyl-pyridin-2-yl)- ethanone
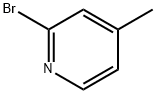
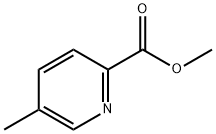
To a solution of 2-bromo-4-methyl-pyridine (5 g, [29MMOL)] in dry THF (70 ml), was added dropwise a solution of sodium bis- (trimethylsilyl) amide 2M in THF (32 ml, 2.2eq) [AT-30°C] under nitrogen. The mixture was stirred [AT-30°C] for 1 h, then 6- methylpicolinic acid methyl ester (4.82 g, 32. 3mmol, 1. [1 EQ)] was added. The reaction mixture was stirred at room temperature overnight. Diethyl ether was added and the precipitated solid filtered and washed with diethyl ether. The solid was diluted with saturated NH4CI solution and the aqueous phase extracted with ethyl acetate. The organic layer was dried over [NA2SO4] and concentrated. The resulting orange powder was washed with pentane to give the title compound as a yellow solid (5.84 g, 70%); [[APCI] MS] [M/Z] 292 (MH+).
References:
WO2004/16606,2004,A1 Location in patent:Page 19
4926-28-7
451 suppliers
$5.00/1g
29681-38-7
110 suppliers
$13.00/100mg

446880-80-4
9 suppliers
inquiry

4926-28-7
451 suppliers
$5.00/1g
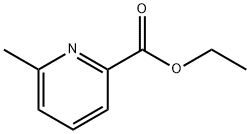
39640-51-2
31 suppliers
$35.00/250mg

446880-80-4
9 suppliers
inquiry