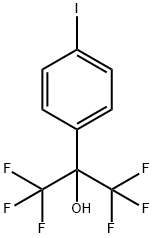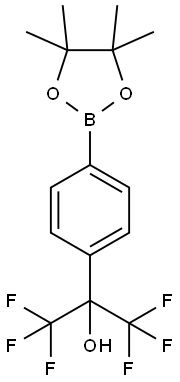
1,1,1,3,3,3-hexafluoro-2-(4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)phenyl)propan-2-ol synthesis
- Product Name:1,1,1,3,3,3-hexafluoro-2-(4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)phenyl)propan-2-ol
- CAS Number:449804-09-5
- Molecular formula:C15H17BF6O3
- Molecular Weight:370.0951
Yield:449804-09-5 45%
Reaction Conditions:
with palladium bis[bis(diphenylphosphino)ferrocene] dichloride;anhydrous potassium acetate in 1,4-dioxane at 90;Inert atmosphere;
Steps:
1.B Procedure 1, Step B.
To a solution of compound Intermediate 1 (1.0 g, 1.0 equiv) in anhydrous l,4-dioxane (20 mL) was added bis(pinacolato)diboron (0.89 g, 1.3 equiv), potassium acetate (0.265 g, 3.0 equiv), and Pd(dppf)2Cl2 (100 mg, 0.05 equiv). The mixture was degassed and then bubbled with N2 for 5 min, and then stirred at 90°C overnight. Upon cooling to room temperature, the mixture was poured into NH4Cl solution and extracted with DCM. The organic phase was dried over MgS04, concentrated, and purified with silica gel column chromatography to afford l,l,l,3,3,3-hexafluoro-2-(4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)phenyl)propan-2-ol (Intermediate 2) (450 mg, yield 45%) as a white solid.
References:
WO2019/177997,2019,A1 Location in patent:Paragraph 0169
722-92-9
127 suppliers
$8.00/1g

449804-09-5
7 suppliers
inquiry