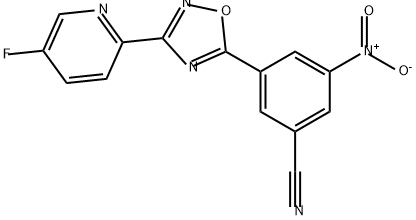
Benzonitrile, 3-[3-(5-fluoro-2-pyridinyl)-1,2,4-oxadiazol-5-yl]-5-nitro- synthesis
- Product Name:Benzonitrile, 3-[3-(5-fluoro-2-pyridinyl)-1,2,4-oxadiazol-5-yl]-5-nitro-
- CAS Number:453566-87-5
- Molecular formula:C14H6FN5O3
- Molecular Weight:311.23
Yield:453566-87-5 131 mg (8%)
Reaction Conditions:
with triethylamine in dichloromethane;N,N-dimethyl-formamide;
Steps:
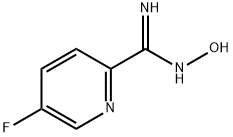
5 3-(5-fluoropyrid-2-yl)-5-(3-cyano-5-nitrophenyl)-1,2,4-oxadiazole
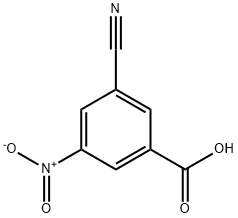
3-(5-fluoropyrid-2-yl)-5-(3-cyano-5-nitrophenyl)-1,2,4-oxadiazole Using the general procedure for the preparation of acid chlorides, 3-cyano-5-nitrobenzoyl chloride was prepared from 3-cyano-5-nitrobenzoic acid (1.0 g, 5.1 mmol). A solution of the acid chloride in dichloromethane (5 mL) at 0° C. was treated with 5-fluoropyrid-2-ylamidoxime (791 mg, 5.1 mMol) and triethylamine (2.1 mL, 15.3 mmol) and then stirred at ambient temperature for 1 hour. The reaction mixture was washed with water and saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated. The residue was dissolved in N,N-dimethylformamide (5 mL) and heated at 110° C. for 4 hours. Standard work up and silica gel chromatography using hexanes:ethyl acetate:dichloromethane (3.5:0.5:4) afforded 131 mg (8%) of 3-(5-fluoropyrid-2-yl)-5-(3-cyano-5-nitrophenyl)-1,2,4-oxadiazole: 1H NMR (CDCl3), δ (ppm): 9.34 (s, 1H), 8.89 (s, 1H), 8.74 (d, 2H), 8.30 (m, 1H), 7.66 (m, 1H).
References:
US2003/55085,2003,A1