
3-CHLORO-N-(3,5-DIMETHOXYPHENYL)-2,2-DIMETHYLPROPANAMIDE synthesis
- Product Name:3-CHLORO-N-(3,5-DIMETHOXYPHENYL)-2,2-DIMETHYLPROPANAMIDE
- CAS Number:454473-71-3
- Molecular formula:C13H18ClNO3
- Molecular Weight:271.74
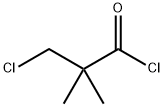
4300-97-4
213 suppliers
$53.00/5G
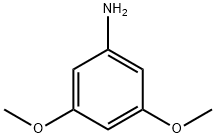
10272-07-8
429 suppliers
$6.00/1g

454473-71-3
12 suppliers
$118.00/500mg
Yield:454473-71-3 46%
Reaction Conditions:
in dichloromethane at 0 - 20; for 13 h;
Steps:
5.1.20. 3-Chloro-N-(3,5-dimethoxyphenyl)-2,2-dimethylpropanamide (substrate for azetidine 20)
3-Chloro-2,2-dimethylpropanoyl chloride (0.42 g, 2.7 mmol) was dissolved in dry CH2Cl2 (15 mL) in a single neck flask and cooled to 0 °C. 3,5-Dimethoxyaniline (0.63 g, 4.1 mmol) in 10 mL of CH2Cl2, was slowly added to 3-chloro-2,2-dimethylpropanoyl chloride solution by dropping funnel. Resulted reaction mixture stirred for 1 h at 0 °C and 12 h at room temperature. After this water (50 mL) was added to reaction flask and stirred for 30 min. Dichloromethane (50 mL) was added to reaction mixture and water layer separated by separating funnel. Dichloromethane layer was washed with 10% HCl (25 mL), and then with water (25 mL). Dichloromethane layer dried over Na2SO4, evaporated on rotary evaporator under vacuo. Resulted white solid was passed through silica gel using CH2Cl2 to obtain pure amide. Rf (2% DCM/MeOH) 0.6. Yield 0.34 g (46%), mp 116-118 °C. 1H NMR (500 MHz, CDCl3) δ 7.42 (br s, 1H), 6.78 (d, J=2.5 Hz, 2H), 6.26 (t, J=2.5 Hz, 1H), 3.78 (s, 6H), 3.69 (s, 2H), 1.40 (s, 6H). 13C NMR (125 MHz, CDCl3) δ 173.1, 161.0, 139.2, 98.4, 97.2, 55.3, 52.6, 45.0, 23.6. IR (plate): 3337, 2935, 2838, 1664, 1618, 1559, 1453, 1418, 1197, 1153, 1068, 828 cm-1.
References:
Balkrishna, Shah Jaimin;Bhakuni, Bhagat Singh;Kumar, Sangit [Tetrahedron,2011,vol. 67,# 49,p. 9565 - 9575] Location in patent:experimental part