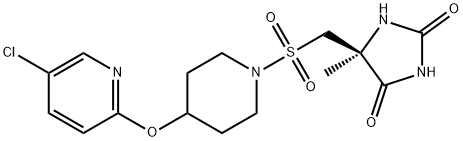
(S)-5-(((4-((5-broMopyridin-2-yl)oxy)piperidin-1-yl)sulfonyl)Methyl)-5-MethyliMidazolidine-2,4-dione synthesis
- Product Name:(S)-5-(((4-((5-broMopyridin-2-yl)oxy)piperidin-1-yl)sulfonyl)Methyl)-5-MethyliMidazolidine-2,4-dione
- CAS Number:459814-89-2
- Molecular formula:C15H19BrN4O5S
- Molecular Weight:447.3042

260441-44-9
29 suppliers
$98.00/100mg
459818-50-9
1 suppliers
inquiry

459814-89-2
11 suppliers
$502.57/5MG
Yield:459814-89-2 71%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in tetrahydrofuran;Isopropyl acetate at -15 - 20; for 4.5 h;Product distribution / selectivity;
Steps:
5.1.b
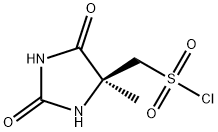
fr) rSV5-r4-(5-Chloro-pyridin-2-yloxyVpiperidine-l-sulfonylmethvn-5-methyl- 30 imidazolidine-2 ,4-dione; Diisopropylethylamine (24.3 mL, 0.139 mol, 1 mol eq.) was added to the iso-PrOAc solution prepared in part (a) [ca. 300 mL; equivalent to 31.2 g, 0.146 mol, 1.05 mol eq. of 5-chloro-2-(piperidin-4-yloxy)-pyridine] in one portion at RT. The solution was then cooled to -15 °C.((S)-4-Methyl-2,5-dioxo-imidazolidin-4-yl)-methanesulfonyl chloride (31.65 g, 0.139 mol, 1 mol eq.) was dissolved in dry THF (285 mL) at RT with stirring. The resulting solution was then added to the iso-PrOAc solution of 5-chloro-2-(piperidin-4-yloxy)- pyridine dropwise at -15 0C over about 1.5 h. A precipitate was seen on addition of the ((S)-4-methyl-2,5-dioxo-imidazolidin-4-yl)-methanesulfonyl chloride. At the end of the addition, dry THF (32 mL) was added to the reaction mixture to wash the line and the mixture was stirred for 1 h at - 15 0C. It was then warmed to 20 °C over 1 h and stirred at 20 °C for 1 h further. The reaction was quenched with 10 wt% NaHSO4 (157 mL) with rapid stirring. After about 15 minutes, the biphasic mixture was allowed to settle, and the bottom aqueous phase was separated and discarded. This acid wash procedure was repeated once more. The organic phase was then washed with water (157 mL) using rapid stirring and allowing complete phase separation before partitioning. The reaction solution was then warmed to 40 °C and washed again with water (157 mL). THF (95 mL) was added to the organic layer that was then warmed to 40 0C and filtered at 40 0C to remove any particulate matter. The solvent volume was then reduced to about 157 mL by reduced pressure distillation with the jacket temperature at 55 °C. zso-PrOAc (317 mL) was then added and the volume was again reduced to about 157 mL. Two more put-and-takes of zs?-PrOAc (317 mL) were carried out. Solids began to precipitate out during the distillations and a suspension resulted. The volume was reduced to about 157 mL each time and after the final distillation a small sample of solvent was then removed from the reaction mixture for residual THF analysis. The 1H NMR showed no THF peaks. The contents of the reaction were then cooled to 0 °C and the product was collected by filtration. The reaction vessel was washed with zs?-PrOAc (63 mL) and this rinse was used to wash the product on the
References:
WO2007/106022,2007,A2 Location in patent:Page/Page column 21; 47-49