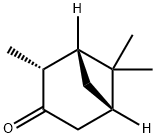
[1R-(1alpha,2beta,5alpha)]-2,6,6-trimethylbicyclo[3.1.1]heptan-3-one synthesis
- Product Name:[1R-(1alpha,2beta,5alpha)]-2,6,6-trimethylbicyclo[3.1.1]heptan-3-one
- CAS Number:473-62-1
- Molecular formula:C10H16O
- Molecular Weight:152.23
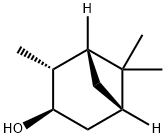
1196-00-5
37 suppliers
$312.00/250g
![[1R-(1alpha,2beta,5alpha)]-2,6,6-trimethylbicyclo[3.1.1]heptan-3-one](/CAS/GIF/473-62-1.gif)
473-62-1
7 suppliers
inquiry
Yield:473-62-1 93.4%
Reaction Conditions:
with Jones reagent in acetone at 0; for 3 h;
Steps:
165
Preparation 165(lR,2R,5S)-2,6,6-trimethylbicyclo[3.1.1]hept; Jones reagent: add a mixture of 9 ml of H2SO4 and 40 ml of H20 to a solution of Cr203 (15.0 g, 0.15 mol) in H20 (20 mL) at 0 °C.Add dropwise the above Jones reagent to a solution of (lR,2R,3R,5S)-2,6,6- trimethylbicyclo[3.1.1]heptan-3-ol (23.1 g, 0.15 mol) in acetone (100 mL) over a period for 2 hours at 0 °C. Stir the resultant mixture for additional 1 hour. Dilute the mixture with 100 mL of water and then extract the aqueous mixture with ether (50 mL X3). The combined organic layers are washed with brine (20 mL), dried over Na2SC>4, filtered, and concentrated under vacuum to give crude (lR,2R,5S)-2,6,6-trimethylbicyclo[3.1.1]heptan-3-one as pale yellow oil (21.3 g, yield: 93.4%), MS (m/z): 151 (M-1).
References:
WO2012/100423,2012,A1 Location in patent:Page/Page column 76-77
![[1R-(1alpha,2beta,3beta,5alpha)]-2,6,6-trimethylbicyclo[3.1.1]heptan-3-ol](/CAS/GIF/2734-31-8.gif)
2734-31-8
1 suppliers
inquiry
![[1R-(1alpha,2beta,5alpha)]-2,6,6-trimethylbicyclo[3.1.1]heptan-3-one](/CAS/GIF/473-62-1.gif)
473-62-1
7 suppliers
inquiry
![Bicyclo[3.1.1]hept-2-ene, 3-iodo-4,6,6-trimethyl-, (1S,4R,5R)-](/CAS/20210305/GIF/911673-20-6.gif)
911673-20-6
0 suppliers
inquiry

7785-70-8
228 suppliers
$21.00/5mL
![[1R-(1alpha,2beta,5alpha)]-2,6,6-trimethylbicyclo[3.1.1]heptan-3-one](/CAS/GIF/473-62-1.gif)
473-62-1
7 suppliers
inquiry
![Bicyclo[3.1.1]hept-2-ene, 6,6-dimethyl-4-methylene-, (1S,5S)-](/CAS/20210111/GIF/190715-28-7.gif)
190715-28-7
1 suppliers
inquiry
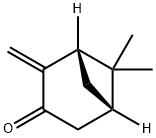
19890-00-7
3 suppliers
inquiry
![[1R-(1alpha,2beta,5alpha)]-2,6,6-trimethylbicyclo[3.1.1]heptan-3-one](/CAS/GIF/473-62-1.gif)
473-62-1
7 suppliers
inquiry
![Bicyclo[3.1.1]heptan-3-one, 2,6,6-trimethyl-, (1R,2S,5S)-](/CAS/20210111/GIF/18492-59-6.gif)